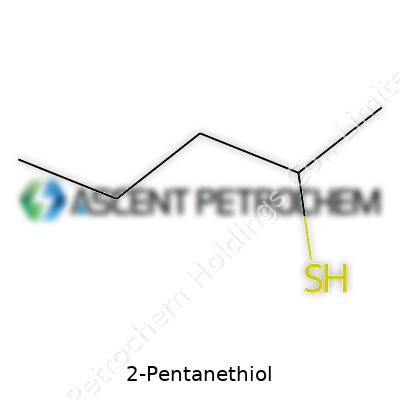
2-Pentanethiol: A Closer Look
Historical Development
Chemists first took real notice of 2-pentanethiol in the wave of early organosulfur research during the twentieth century. This compound came from a lineage of thiols, substances once known mostly for their sharp, unpleasant smell and their habit of showing up in crude oil and skunk spray. Back in the 1950s, as oil refining picked up pace, industrial laboratories documented 2-pentanethiol while mapping out sulfur-bearing molecules in fuel. That early documentation laid the groundwork, but it took significant improvement in distillation and chromatography before scientists could isolate the compound with any useful degree of purity. Modern chemical engineering now produces 2-pentanethiol with a level of quality that has opened up a broad range of applications, including research, industry, and flavor chemistry.
Product Overview
2-Pentanethiol turns up as a clear to slightly yellow oily liquid, carrying a strong, skunk-like odor that makes its presence known even in small concentrations. Laboratories and companies work with it in bulk for synthesizing specialty chemicals, fine-tuning flavors, and prepping intermediates in pharmaceuticals and agrochemicals. At its core, this molecule stands out for its reactive thiol group, which marks it as valuable and challenging to handle all at once. Suppliers today usually ship it in tightly sealed metal or glass drums, with clear labeling and paperwork to track every step from production to lab bench.
Physical & Chemical Properties
2-Pentanethiol, with the molecular formula C5H12S, flows as a low-viscosity liquid at room temperature. It boils at roughly 119°C and registers a melting point around -90°C. With a density close to 0.83 g/cm³, lighter than water but heavier than gasoline, it floats on surfaces in liquid mixtures. Its solubility in water remains low, yet mixes easily with most organic solvents such as hexane or benzene. From a chemical standpoint, the molecule reacts quickly with oxidizing agents and gives off flammable vapors. The distinctively strong, sulfurous aroma sticks not only to glassware but also to memories of anyone who’s handled it in a fume hood.
Technical Specifications & Labeling
Every shipment of 2-pentanethiol requires a clear list of technical specs. Purity levels usually clock in above 98% for research applications. Labels carry vital details: UN number as a hazardous liquid, CAS number 2084-19-7, complete supplier information, and pictograms to flag its flammable, harmful, and environmentally unsafe character. Maximum allowable residual solvents, water content, and trace impurities such as other thiols or alcohols get checked at every batch. Chemical safety datasheets bring attention to the compound’s personal protection requirements, accidental release procedures, and first-aid steps for exposure.
Preparation Method
Industrial production takes one of two main routes. Traditional methods rely on alkylation — for example, passing 2-chloropentane over sodium hydrosulfide to swap out a chlorine atom for a thiol group. Careful conditions, such as gentle heating and inert atmospheres, keep unwanted byproducts at a minimum. Another approach uses radical addition of hydrogen sulfide to 1-pentene with a catalyst, which reduces waste but demands expensive setup. Both methods need sharp control of sulfur sources and temperatures, as escapes or side reactions can push costs up and make purification more difficult.
Chemical Reactions & Modifications
2-Pentanethiol stands out for its reactive sulfur group. It bonds easily to soft metals, making it handy in surface chemistry or the creation of metal-organic frameworks. Air and oxidizing agents convert it into the corresponding disulfide — a reaction useful to chemists but a cause of product instability in poorly sealed containers. Researchers also rely on its readiness to react with alkyl halides and acyl chlorides; such modifications shape the building blocks for agrochemicals, fragrances, or specialized lubricants. For flavor chemists, mild oxidation produces key aroma compounds found in both synthetic and natural blends.
Synonyms & Product Names
A chemist might refer to this compound as 2-pentyl mercaptan, sec-amyl mercaptan, or simply 2-pentanethiol depending on the context. Catalogs and safety sheets may list “pentan-2-thiol” under IUPAC rules. Each synonym tells something about its origins and structure — "mercaptan" being the old-school term for thiols. Over time, the industry settled on 2-pentanethiol for clarity, especially when working across regulatory and supply chains.
Safety & Operational Standards
Work with 2-pentanethiol always calls for good ventilation and properly rated protective gear: gloves, goggles, lab coats, and vapor masks. This compound ignites at low flash points, so open flames and static discharge must stay far away. Fume hoods remain mandatory, with special care in waste handling to trap vapors and stop spills from seeping into drains or soil. Emergency shower and eyewash stations need to remain within arm’s reach since liquid exposures can irritate skin and eyes. Facilities handling significant quantities must have spill kits and fire extinguishing agents specifically rated for volatile organosulfur chemicals. Safety training never becomes routine or optional with these materials.
Application Area
A surprising range of industries makes use of 2-pentanethiol. The flavor and fragrance world, despite the strong base odor, adds tiny amounts to complex notes imitating whiskey, cheese, or tropical fruit where a sulfur tang brings authenticity. Chemical manufacturers depend on it for reactions in agrochemicals, plastics, and pharmaceutical precursors. Oil and gas professionals keep it on hand for training and calibration, since even trace quantities can simulate natural gas leaks for safety drills. Some research groups harness its reactivity to tweak surface properties of metals or carbon nanomaterials, putting a thiol "hook" onto otherwise slippery surfaces.
Research & Development
Research continues to push into the chemistry of thiols like 2-pentanethiol. Labs now study how to control sulfur transfer more efficiently, aiming for greener and safer transformations. On the material side, recent work taps 2-pentanethiol for customized surface coatings, sometimes to make medical devices or sensors more sensitive or less prone to fouling. Environmental chemists look at ways to break down thiols in waste streams, curbing their escape into air or water. As analytical equipment improves, trace studies even explore how 2-pentanethiol forms in food or decomposes in the environment, mapping out where and how these molecules affect health and quality.
Toxicity Research
Toxicologists recognize organosulfur compounds as potent — both for their effects on the nervous system and for their environmental persistence. Animal models exposed to 2-pentanethiol show behavioral and metabolic changes at moderate doses. For humans, the main risks come from inhalation, with symptoms ranging from dizziness and eye watering to headaches and nausea. Skin contact can bring on dermatitis or allergic reactions, and no one forgets the persistent smell that clings for days. Environmental risks include toxic hits to aquatic organisms and trouble for sewage treatment bacteria. Ongoing research tries to clarify safe exposure limits, but the advice tends to favor strict controls and avoidance wherever possible.
Future Prospects
The coming years will likely see 2-pentanethiol drawing more interest for specialty applications. As green chemistry gains traction, finding production pathways that generate less hazardous byproducts matters more. Stricter environmental rules push industry toward tighter containment and better waste processing. New uses could open up in nanotechnology, where thiol-reactive surfaces unlock clever sensor systems and targeted drug delivery devices. Biotechnology may borrow from traditional organosulfur chemistry to create novel molecules for health and agriculture, always with careful attention to both benefit and hazard. For those in labs and factories, the next round of developments will start at the intersection of old chemistry know-how and new ideas for sustainable progress.
The Role of 2-Pentanethiol in Modern Industry
A lot of folks probably haven’t heard much about 2-pentanethiol unless chemistry played a big part in their schooling or work life. In short, it's a sulfur-containing organic compound known for its strong, often pungent odor. We rarely meet this chemical in its pure form in daily life, but it pops up in a good number of places people care about—mainly behind the scenes.
From Flavors to Fuels: Where It Matters
2-Pentanethiol shows up as a flavor and fragrance ingredient. Chemists lean on thiols to build certain “meaty” or “savory” notes in artificial food flavorings. If you’ve ever had processed foods, pungent cheese, or seasonings, some of their unique aroma can trace back to these sulfur-based molecules. Although the amounts used in flavors remain tiny, they help round out the complexity of certain tastes.
Industrially, 2-pentanethiol’s value extends beyond food. It plays a part in making specialty lubricants. Adding this compound can tailor lubrication properties, especially in scenarios where protection against wear and extreme environments matters. Automobile and machinery manufacturers need these specialty blends for engines and moving parts. Without proper additives like 2-pentanethiol, efficiency drops off, and expensive breakages become more common.
Expert Opinions and Health Concerns
Scientists pay close attention to safety whenever using chemicals like thiols. Exposure to 2-pentanethiol, even at low levels, can cause headaches, nausea, or irritation. Chemical processing plants and labs rely on ventilation and personal protective equipment to keep workers safe. Strict guidelines from OSHA and the EPA keep the risks under control.
Outside the workplace, folks rarely come face to face with 2-pentanethiol. Production sites take special measures to capture and neutralize fumes, since the odor carries a long way. The sulfur smell acts as a warning sign that something needs fixing, which sometimes proves helpful during leak checks. Strong-smelling chemicals have saved plenty of people by sounding the alarm before more damaging exposures occur.
Environmental Considerations
Sulfur compounds never get a free pass when it comes to environmental safety. Leaks or improper disposal threaten water sources, and the strong smell sticks around. Treatment plants use scrubbers and biological filters to clean up air emissions from factories. Experience tells me that plants with rigorous training programs and good maintenance records handle these jobs more safely.
Waste treatment remains a community issue since emissions and runoff tend to affect neighborhoods nearby. Some industrial facilities have faced fines and lawsuits after odors or spills reached residential areas. Open communication and investment in odor-control technology help keep everyone on better terms. Knowing that your local plant takes community safety seriously lets people rest a little easier.
Future of 2-Pentanethiol in Everyday Products
As more companies focus on green chemistry and reducing toxic profiles, alternative compounds sometimes replace older chemicals. Still, 2-pentanethiol fills key roles that not every substitute can handle as well. Researchers work on safer handling methods and look for ways to recycle or neutralize residues from industrial use.
People rarely think about the science and engineering that go into the flavors we recognize or the durability built into everyday machines. Still, all it takes is a single missing ingredient or mishandled chemical to make folks pay attention. Keeping experts involved, listening to worker concerns, and holding companies accountable all help make the benefits of 2-pentanethiol outweigh the risks.
The Formula and the Basics
2-Pentanethiol comes with the chemical formula C5H12S. That boils down to five carbon atoms, twelve hydrogen atoms, and one sulfur atom. Move the sulfur along the pentane chain, you get different isomers. In 2-pentanethiol, the sulfur links to the second carbon, which changes the way the whole molecule behaves and smells. If you've ever worked with similar thiols, you know they usually assault your senses—a sharp, strong odor that sticks with you long after you’ve capped the bottle.
Why Structure Matters
Organic chemistry reminds us that even a small shift in structure (like moving sulfur from the first to the second carbon) changes properties in obvious and subtle ways. The body doesn’t react to 2-pentanethiol the way it might to pentanethiol where sulfur is on the first carbon. That comes back to chemical interactions with cells, receptors, and enzymes. Anyone who has been in a working lab knows how important it is to double-check which isomer they're holding—one wrong assumption can wreck experiment results, not to mention safety protocols.
Everyday Impact and Personal Experience
2-Pentanethiol crops up in unexpected places. Industrial labs use it for synthesizing flavors, so the odd tang in some artificial foods and drinks sometimes owes a bit to this compound. Its sharp, skunky scent shows up in flavoring agents, and people like me, who’ve spent time researching food additives, understand the challenge of balancing effectiveness as an odorant with regulations on volatile sulfur compounds. Too much, and you spoil a batch. Too soft and you lose the effect.
Health, Safety, and the Human Element
Inhaling volatile thiols comes with risks. 2-Pentanethiol, like its cousins, can irritate your nose, eyes, and throat. Even a small spill can clear out a room. Experience has taught many chemists—myself included—the importance of good fume hoods and personal protective equipment. Exposure aside, proper labeling and storage stay critical because sulfur-containing chemicals often spark confusion on the shelf, especially if containers aren’t clearly marked. An unplanned reaction or cross-contamination can turn routine work into an emergency.
Solutions for Real-World Concerns
Handling chemicals like 2-pentanethiol safely doesn’t only sit with the worker at the bench. Manufacturers, labs, and regulatory bodies all play a role in improving labeling, training, and waste management. I remember a story from a university lab where a mislabeled bottle led to an accidental release; nobody got hurt, but the cleanup took hours, and the lesson stuck. Upstream regulation, rigorous in-house audits, and open communication help prevent these situations before they even start.
Looking Forward: Smart Use and Education
With new advances in monitoring and digital labeling, more workplaces catch mistakes before they escalate. Workshops and hands-on training replace old, printed manuals. New chemists get a sense of real-world dangers, not just textbook warnings. People who learn on the job respect the difference a single atom can make in a molecule like 2-pentanethiol. As synthetic methods grow, chemical safety—rooted in experience and clear understanding—matters more than ever.
Why 2-Pentanethiol Calls for Respect
2-Pentanethiol is one of those pungent chemicals you do not forget after you have worked with it once. If you have ever caught a whiff, you know—the smell hits fast and hard. This compound, like other thiols, can get under your skin or into your lungs before you realize you have been careless. Beyond the sharp odor, 2-Pentanethiol brings real risks: skin burns, eye irritation, and nasty headaches that do not let up quickly. Spending time with volatile organosulfur chemicals through years in the lab, I learned early that gloves and goggles are never optional.
Personal Protective Gear: Your First Line of Defense
Every time you even think about unscrewing a bottle of 2-Pentanethiol, reach for the nitrile gloves, lab coat, and chemical splash goggles. Latex does not hold up—organic solvents and sulfur compounds sneak through before you notice. If you believe standard glasses or lightweight gloves are good enough, the stinging eyes and burning skin will teach you otherwise after just one mistake.
For those transferring larger amounts or running reactions using this substance, go with a full-face shield and heavy-duty apron. Bugs me to see people skipping the basics because “it’s just a quick transfer.” Quick transfers turn messy fast when thiols spill. Old-timers around me in the lab avoided those nasty headaches by always handling strong-smelling liquids inside a fume hood, never in open air.
Keep It Contained: Ventilation and Workspaces
A strong exhaust system matters more with thiols than almost anything else. Just opening a cap of 2-Pentanethiol can stink up a whole floor if it wafts past a door. Fume hoods are not there for show—they keep the vapor away from your nose and out of your lungs. Good ventilation cuts down on the risk of headaches, dizziness, or accidental inhalation of vapors.
If you do spill something, make sure you have the right materials on hand. Absorbent pads soaked in an oxidizer (like bleach solution) help neutralize and mask the smell, and hydraulic cement packing over drains keeps anything from soaking into lab floors. Good ventilation keeps headaches and accidental exposure from becoming part of your everyday work.
Proper Handling and Emergency Steps
Never eat or drink anywhere near this material. Accidentally touching your face with contaminated gloves or hands will remind you why trainings warn against it. I have seen colleagues with chemical burns on their lips from a careless lunch break after a lab session.
If things go sideways, water and gentle soap are your friends. Wash skin immediately—do not wait until the tingling turns to burning. For splashes, eyewash stations and safety showers need to stay clear and accessible. Time is the difference between a minor burn and days of discomfort.
Storing 2-Pentanethiol demands secure, upright containers in well-ventilated, cool spaces—far from heat sources. Even small leaks fill rooms with an overpowering stink. Upright bottles, tight lids, and frequent checks for crusted caps or residue around seals keep these problems down.
Solid Practices: Training, Labels, and Waste
Labels need to spell things out in plain words. No half-faded notes or cryptic abbreviations. Anyone walking into storage or a shared lab must know what's in a bottle before opening it. Regular audits and drills help new folks learn the ropes and stay alert.
Disposing of 2-Pentanethiol waste? Specialized chemical waste containers only—never toss it down the drain, even if you think it’s been diluted. Water systems pick up that rotten egg stench and share it with the next building over.
Moving Toward a Safer Lab
Getting careless with thiols leads to long-lasting regrets. Anyone handling 2-Pentanethiol must treat every step with respect. Strong smell, serious hazards—these things stick with you long after the last bottle is put back on the shelf. Solid work practices, protective gear, clear labeling, and respect for the risks carve out a safer place to work and come home in one piece. Newcomers may roll their eyes at the layers of protection, but experience proves that you never get used to chemical burns or migraines from exposure. In the long run, smart precautions let you focus on your research instead of your mistakes.
The Nose Knows: Living with Unpleasant Smells
Walking into a lab that sees real, regular work with organosulfur compounds gets a person’s attention in a way that other workplaces don’t. Few smells in chemistry stick to your memory—or your clothes—the way sulfur compounds can. 2-Pentanethiol really leaves an impression. People sometimes ask if a strong, rotten odor comes from a gas leak or a forgotten lunch. In truth, the sharp, deeply unpleasant scent likely signals a thiol at work, and 2-Pentanethiol stands right among the most memorable offenders.
Chemists tend to joke about “stinky chemistry,” but the situation can feel serious. 2-Pentanethiol doesn't just bring a faint whiff of something odd; it sinks into the air and stays there. The odor reminds many of rotting cabbage, decomposing onions, or the distracted skunk that crossed the road last summer. It’s powerful at a distance and completely overwhelming up close. Colleagues avoid the area, and even after cleaning up, the memory lingers. Wearing gloves and masks helps, but experience shows that even with careful handling, the tiniest accidental spill means a lingering mark on clothing, skin, and even hair.
The Science Behind the Stench
There’s a good reason for this strong reaction. Human noses can pick out sulfur compounds in extremely low concentrations—sometimes just a few parts per billion. That sensitivity keeps people safe, since nature often ties these odors to danger, spoilage, or toxicity. Thiols, like 2-Pentanethiol, show up naturally in skunk spray, bad breath, and decomposing plant matter. In a lab setting, even a sealed bottle releases a hint of odor when opened. The molecular structure of 2-Pentanethiol includes a sulfur-hydrogen group, and this feature interacts powerfully with smell receptors. That’s not just a quirky feature of the compound; it’s a simple fact of chemistry—sulfur punches through most other scents and leaves its own mark.
Why It Matters in Industry and Society
Even so, chemistry labs and industry rely on thiols. 2-Pentanethiol finds use from pharmaceuticals to custom chemical synthesis. Managing its smell becomes a workplace priority. Good fume hoods and careful storage stop the spread of noxious air throughout entire buildings, but older facilities and crowded bench spaces sometimes struggle. People working with thiols often share advice: double-bag any waste, don’t take lab coats home, clean up immediately, and warn housemates or neighbors if a big job requires opening a large container.
The real health risks with 2-Pentanethiol come from inhalation at high concentrations, and nobody wants to be exposed more than needed. Regular air monitoring, tightly written safety procedures, and quick communication build a safer environment. On top of that, ventilation and specialized filters actually do measurable good. Trusting your nose and listening to your co-workers’ reactions make a real difference—there’s no substitute for experience in spotting a spill or accidental release.
Making Life With Strong Smells Tolerable
If a strong odor like this sticks around even after cleaning, remedies include airing out the space, using activated charcoal, and sometimes clothing changes. In the worst cases, sulfur scents can drive people out of rooms. Even so, respect for workplace safety culture grows from the collective effort to keep these “chemical scars” from ruining both spaces and moods. Thiols leave an impression on anyone who works with them—and on every nose they meet.
Understanding 2-Pentanethiol
Anyone who’s worked in a chemistry lab has probably run across thiols. These sulfur-containing compounds come with a smell that’s hard to forget, sort of like rotten onions or garlic. The structure of 2-pentanethiol—five carbons long, with a sulfur atom thrown in—gives it an oily quality. Some folks who deal with chemicals, especially those in flavor and fragrance work, might expect it to handle water about as well as other small molecules. Still, experiences in the lab show something different.
What Happens When You Mix 2-Pentanethiol with Water?
Pouring a bit of 2-pentanethiol into water and giving it a shake leads to two layers, not one clear solution. That familiar oil-on-water effect shows up fast. This isn’t just about the shake—it goes back to molecular structure. Water, which loves to hydrogen bond, hardly notices the heavy, oily tail of 2-pentanethiol. The sulfur at the end holds on to its own kind, not to water. Methanol and ethanol—smaller cousins in the alcohol family—mix right in, but add just a few more carbon atoms, as in 2-pentanethiol, and the story changes.
Basic data backs this up. Solubility records put 2-pentanethiol's ability to mix with water at less than 0.1 grams per 100 milliliters. Some reference tables go so far as to call it “insoluble,” which lines up with what you see and smell. The slight part that does mix gives off a strong, stinky punch—enough to alert passers-by to its presence and highlight why proper ventilation matters. The strong odor comes from vapor above the water where those molecules hang out.
Why Does This Matter?
It’s easy to think a chemical’s solubility doesn’t mean much unless mixing drinks or working on a new pharmaceutical. That’s not the case here. Many emergency room visits and workplace accidents link to chemicals behaving in ways people didn’t expect. A liquid that won’t mix with water can make cleanup trickier, especially in the event of a spill. Water may spread the chemical out instead of washing it away. This also has consequences for the environment. If 2-pentanethiol leaks into a river, most of it floats on the surface or sticks to organic matter, rather than dissolving.
For industrial users, this poor water solubility can mean headaches. Removing traces from clothing or equipment often takes more than soap and water. Specialized detergents or organic solvents do a better job. Proper storage, handling, and spill response training follow from knowing a substance won’t just rinse away.
Steps Toward Safety and Better Practice
Anyone using 2-pentanethiol can keep trouble at bay with a clear approach. A written spill protocol goes a long way—absorbent pads designed for oils grab most of the chemical. Fume hoods, nitrile gloves, and eye protection protect from that strong odor and possible health risks. It also makes sense for industries to train new hires in chemical handling, not just for compliance but to drive home lessons learned the hard way.
On the environmental front, the only real fix comes from catching leaks early and storing thiols securely. Proper waste disposal plans ensure these strong-smelling compounds don’t reach drains or streams. This approach keeps neighbors happier, too, as those pungent aromas don’t exactly blend into the background.
Final Thoughts
For anyone mixing, storing, or cleaning up after 2-pentanethiol, understanding its poor relationship with water isn’t just chemistry trivia. It’s an everyday concern for health, safety, and the good of the community. Knowing how a substance behaves lets you work smarter and avoid headaches—sometimes literally.
| Names | |
| Preferred IUPAC name | pentane-2-thiol |
| Other names |
Amyl mercaptan
Amylthiol 1-Ethyl-1-propanethiol Pentane-2-thiol |
| Pronunciation | /tuːˌpɛn.teɪnˈθaɪ.ɒl/ |
| Identifiers | |
| CAS Number | 110-66-7 |
| Beilstein Reference | 1209225 |
| ChEBI | CHEBI:86416 |
| ChEMBL | CHEMBL181806 |
| ChemSpider | 67138 |
| DrugBank | DB14005 |
| ECHA InfoCard | 08bc59b5-53f8-4b5c-8e96-59406b0d3e2c |
| EC Number | 211-185-8 |
| Gmelin Reference | 7432 |
| KEGG | C02573 |
| MeSH | D010419 |
| PubChem CID | 10947 |
| RTECS number | SK8025000 |
| UNII | Z8S148G2WT |
| UN number | UN2391 |
| Properties | |
| Chemical formula | C5H12S |
| Molar mass | 120.24 g/mol |
| Appearance | Colorless liquid |
| Odor | unpleasant, garlic-like |
| Density | 0.832 g/mL at 25 °C (lit.) |
| Solubility in water | Insoluble |
| log P | 2.38 |
| Vapor pressure | 1.9 mmHg (at 25°C) |
| Acidity (pKa) | 10.8 |
| Basicity (pKb) | pKb = 3.77 |
| Magnetic susceptibility (χ) | -65.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.444 |
| Viscosity | 1.740 mPa·s (25 °C) |
| Dipole moment | 1.66 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 212.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -108.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3367.7 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS06, GHS07, GHS08 |
| Pictograms | GHS06,GHS08 |
| Signal word | Warning |
| Hazard statements | H226, H302, H311, H315, H318, H331, H335, H412 |
| Precautionary statements | Precautionary statements of 2-Pentanethiol: "P210, P261, P273, P280, P301+P310, P303+P361+P353, P304+P340, P311, P321, P330, P361, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | 2-2-2-W |
| Flash point | 38 °C |
| Autoignition temperature | 215 °C |
| Explosive limits | 1.1% - 6.7% |
| Lethal dose or concentration | LD50 oral rat 132 mg/kg |
| LD50 (median dose) | LD50 (median dose): 300 mg/kg (oral, rat) |
| NIOSH | SNM |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-Pentanethiol: "1 ppm (skin) |
| REL (Recommended) | 0.5 ppm |
| IDLH (Immediate danger) | 400 ppm |
