3,3'-Dithiodipropionic Acid: A Deep Dive Into Its Science and Industry Impact
Historical Development
Long before 3,3'-Dithiodipropionic acid became a familiar name in labs and factories, the pursuit for effective thiol-containing compounds gave rise to its discovery. Early sulfur chemists in the mid-20th century hunted for molecules that could bridge biological systems and industrial synthesis. I remember coming across literature that tracked this acid's roots to early polymer research, where scientists sought tough, flexible, and safe links between organic molecules. This compound showed up in patents and theses, often associated with advancements in rubber processing and protein cross-linking. Its history rarely gets the spotlight, yet labs relied on its properties for decades before it appeared in wider industrial circles.
Product Overview
You can spot 3,3'-Dithiodipropionic Acid in the inventory of chemical suppliers targeting pharmaceuticals and plastics. It carries the formula C6H10O4S2 and stands out with its stable disulfide bridge connecting two propionic acid arms. Researchers usually call for high-purity grades since cross-contamination with similar acids affects both safety and effectiveness in experiments. As a result, companies listing this compound focus on clearly labeled synthesis origins, targeted purity levels, and logistics that meet strict storage guidelines.
Physical & Chemical Properties
It appears as a white, crystalline powder, slightly soluble in water but far more comfortable in organic solvents. Experienced chemists recognize the faint, slightly pungent odor — a telltale sign of sulfur content. Its melting point usually hovers between 124 to 126°C, which remains steady under lab conditions. Acidity ranks slightly weaker than acetic acid, yet strong enough to take part in most esterification or acidolysis reactions. The disulfide bond at the core shows resilience until you bring in strong reducing or oxidizing agents. Flammability and low volatility give handlers some relief, and standard chemical reference texts line up its values with reliable consistency.
Technical Specifications & Labeling
Reliable suppliers provide details down to decimal values: purity of at least 98%, clear batch numbers, and analytical proof from NMR and IR spectra. Designers of technical data sheets emphasize not only quality but also safe handling — listing storage in dark, cool areas, away from incompatible bases or acids. Labels usually include common hazard codes, package weights, recommended shelf life, and intended application sectors. From experience, missing or vague labeling signals shortcuts in quality assurance and raises red flags for large-scale buyers.
Preparation Method
Most producers stick to the well-trodden path: oxidative coupling of thiol-containing precursors like β-mercaptopropionic acid under controlled temperature and atmosphere. Some labs experiment with direct oxidation using hydrogen peroxide, but that approach requires close attention to avoid over-oxidation. The industrial method usually favors a mild catalyst, keeping yields high and byproducts minimal. After oxidation, the mixture undergoes crystallization and repeated washing to remove impurities. Equipment must resist corrosion since both reactants and products act aggressively on most metal surfaces. Synthetic challenges revolve around optimizing yield, reducing waste, and cutting down on water use — concerns that scale with production.
Chemical Reactions & Modifications
The standout feature — a disulfide bond — invites both organic and inorganic chemists to break or reorganize this bridge. In labs, tris(2-carboxyethyl)phosphine (TCEP) slices it open, helping link 3,3'-Dithiodipropionic acid to other molecules. The acid groups participate in standard coupling reactions, forming esters or amides. I've watched teams use alkali to tweak the acid's solubility, letting it flow better into water-based mixtures. Its reactivity paves the way for developing bioconjugates, smart polymers, and even self-healing materials, where reversibility of disulfide bonds bridges traditional chemistry with emerging tech.
Synonyms & Product Names
Scientists and cataloguers often use alternative names, including 3,3'-Thiodipropionic acid, Dithiodipropionic Acid, or DTDP acid. Some refer to it as Bis(3-carboxypropyl)disulfide, echoing its IUPAC naming tradition. Commercial suppliers, especially outside North America, lean on local translations and trademarks. From time to time, I encounter errors originating from mislabeling, so it's critical to double-check molecular weights, structures, and CAS numbers — 1119-62-6 ties directly to the correct compound.
Safety & Operational Standards
Handling protocols grew out of lessons learned from repeated near-misses. Even though 3,3'-Dithiodipropionic acid avoids extreme toxicity, it shouldn’t be inhaled, and it demands gloves, goggles, and tight storage. In the plant, ventilation systems get regular checks, limiting the escape of dust or vapor. MSDS sheets flag moderate risks around skin irritation. Disposal aligns with local legislation for organic acids and sulfur-containing wastes, often requiring neutralization before sending out for controlled incineration. Workers know that taking unnecessary chances leads to avoidable hazards; regular trainings keep accident rates low.
Application Area
Its reach kicked off in rubber accelerators and antioxidants, where it helps stabilize products and prevent degradation under stress. Over time, researchers found new ground in biodegradable plastics, coating agents, and drug delivery frameworks. Biochemists rely on it for reversible protein cross-linking, especially in antibody conjugation or controlled release systems. The food packaging sector takes an interest, hoping to leverage the acid’s antioxidant properties for safer, longer-lasting storage films. In my work, demand often correlates with rising bio-based polymer development and health care innovations, reshaping expectations for specialty chemicals.
Research & Development
Academic and commercial labs treat 3,3'-Dithiodipropionic acid as a springboard for new research. Molecular scientists chase pathways for redox-responsive materials, where the disulfide linkage acts as a molecular switch. Environmental projects focus on its degradability, tying its fate in soil to broader questions of microplastic pollution and green chemistry. Advanced medicine uses the compound to calibrate slow-release capsules or tracers. Patents and publications consistently highlight refinements in synthesis, cutting down waste streams and expanding biocompatibility, a sign that innovation rarely slows in this field.
Toxicity Research
Ongoing toxicological studies show it doesn’t register as a persistent environmental toxin, giving users some reassurance. Acute exposure tends to cause skin or eye irritation, but chronic effects appear limited based on current animal testing. Still, regulators keep close tabs on data around reactivity and bioaccumulation, particularly in aquatic systems, because even mild organic acids can disrupt fragile ecologies given enough concentration. It’s clear that comprehensive, peer-reviewed toxicity profiles give industry and consumers assurances that product safety isn’t just an afterthought.
Future Prospects
Whatever lies ahead, the interest in biodegradable and smart materials looks set to drive investment in 3,3'-Dithiodipropionic acid. Companies search for ways to build self-healing or recycling-friendly polymers, leveraging the unique chemistry of disulfide bridges. Pharmaceutical companies track its use in next-generation drug carriers and bio-conjugates, betting on the versatility and mild toxicity profile. Researchers still face old obstacles: scale-up, cost management, and lifecycle analysis. Even so, each step brings the promise of safer, smarter, and more sustainable products built on the backbone of organic sulfur chemistry.
The Chemistry That Connects Ideas to Reality
3,3'-Dithiodipropionic acid doesn’t show up often in everyday conversation, but its value reaches across several corners of modern chemistry. This compound, known for having two carboxylic acid groups linked by a disulfide bridge, often steps in where scientists and manufacturers need a bit of molecular flexibility. Most uses come from its ability to create or break sulfur-sulfur bonds, something that hints at both the fragility and strength found in many life science and material applications.
Building Blocks in the Lab
Synthetic chemists turn to 3,3'-dithiodipropionic acid when creating polymers that need to break apart on cue, like specialized drug delivery materials or biodegradable plastics. For a few years, I watched research teams assemble delivery vehicles for cancer drugs, and controlling degradation time mattered as much as getting the drug to its target. This acid played a big role in those timelines. The disulfide bonds snap under reducing conditions, common in living tissues or inside cells. That “trigger” makes it easier to design materials that stick around until they meet the right conditions, then break down and release what they carry.
Boosting Stability and Reactivity in Materials
Rubber makers and manufacturers often reach for 3,3'-dithiodipropionic acid when they need to improve the toughness and flexibility of their products. The compound acts like a bridge, tying together different strands in a rubber matrix. Auto parts, seals, and even the soles of specialized footwear sometimes owe their performance to sulfur-containing cross-linkers like this one. Tests show that sulfur links help materials stretch farther before snapping, a plus in safety-critical parts.
Playing a Key Role in Antioxidant Research
The food and pharma industries face daily battles against oxidation—no one wants to buy brown apples or stale bread. 3,3'-dithiodipropionic acid functions as an antioxidant. It works by trapping free radicals that trigger the “rusting” of fats and oils. Some labs run experiments comparing its effects with traditional preservatives like BHT and ascorbic acid. Reports show that products containing dithiodipropionic acid last longer on the shelf and develop fewer off-flavors.
Environmental and Safety Factors
While 3,3'-dithiodipropionic acid opens doors for innovation, it pays to keep an eye on safety and disposal. The chemical world has seen enough examples where beneficial additives later caused headaches—think of early flame retardants or plasticizers. Research on sulfur-based compounds includes regular checks for environmental persistence and bioactivity. Current evidence does not flag it as a top environmental risk, but labs and factories handle it with gloves and goggles all the same. That’s just good lab sense and minimizes unplanned exposures.
Looking Ahead
Scientists sometimes chase the next breakthrough by tweaking classic molecules like 3,3'-dithiodipropionic acid. Newer versions build in even more precise triggers, improving performance and safety. For people working on sustainability, these kinds of “smart” components push both technology and policy forward. As demand grows for smarter, safer, and more flexible materials, the lessons from this acid’s uses could shape what comes next on the workbench and in the marketplace.
Breaking Down the Molecular Composition
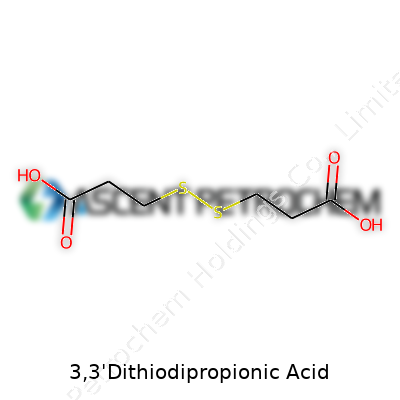
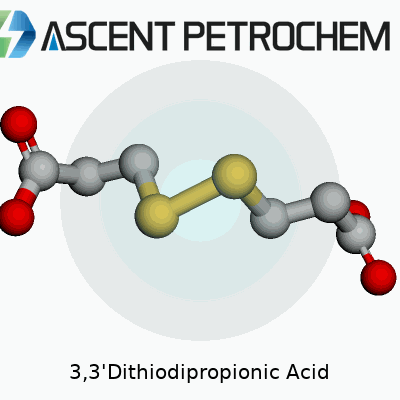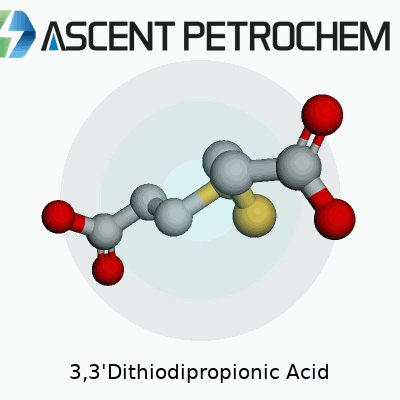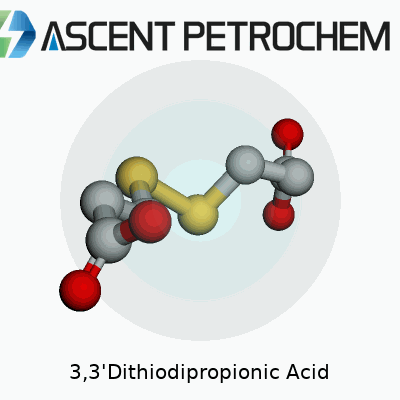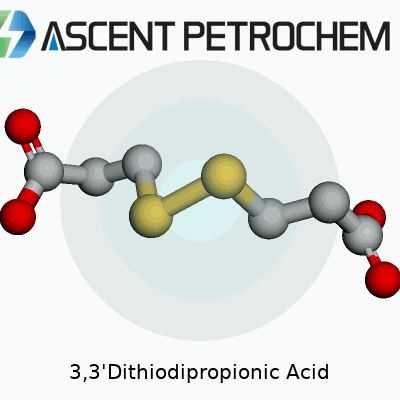
Every molecule tells a story. Look at 3,3'-Dithiodipropionic acid and you find a backbone built from carbon, hydrogen, oxygen, and sulfur. The skeleton looks a bit like two propionic acid groups joined by a sulfur-sulfur bridge. Chemists draw it as HOOC–CH2–CH2–S–S–CH2–CH2–COOH. That means each end holds a carboxylic acid group, recognized for their tendency to interact with water and act as binding points in a variety of reactions.
Sulfur atoms sitting in the center of the molecule link the two chains. This sulfur-sulfur bridge (a disulfide bond) catches the eye, as it offers a crucial reactive site. In real-world chemistry, these sulfur bonds give the compound unique properties, from redox reactivity to the ability to help link proteins or serve in materials science. Simple experiments with this compound highlight both the beauty of symmetry in molecules and the powerful influence of adding sulfur into a structure dominated by carbon-based acids.
Why Structure Matters in Practical Terms
Every chemist learns early on that structure shapes behavior. The carboxylic acid groups in 3,3’-Dithiodipropionic acid grant it the ability to dissolve in polar solvents and engage in hydrogen bonding. That's more than textbook trivia — in practice, these features make the compound valuable in applications that demand reliable solubility and predictable chemical interactions. The disulfide bond nestled between the alkyl chains can break and reform under the right conditions. This redox activity opens the door for it to act as a cross-linking agent or participate in antioxidant processes.
Industry leans on these structural tricks. I remember projects where this type of molecule appeared in experimental antioxidant blends for plastics. The disulfide bond worked like a molecular switch, helping to resist attack from free radicals and extending the useful life of polymers exposed to harsh environments. Researchers tuning hydrogels and protein chemistry also appreciate how these bonds behave under reducing or oxidizing conditions.
Looking Towards Safe Handling and Innovation
Chemistry gives us tools, but handling materials with sulfur always brings a reminder: respect for both the molecule and the potential hazards. Compounds with disulfide bridges sometimes give off unpleasant odors or react strongly with reducing agents. Proper storage in tightly sealed containers, a well-ventilated workspace, and protective gear change a risky procedure into a routine one. Safety data covers details, but a lifelong respect for unfamiliar molecules keeps incidents at bay.
Innovation often sparks from understanding the nuts and bolts of even the simplest compounds. Researchers now look at disulfide-containing acids in biomedical devices, seeking new ways to engineer smart materials or deep-clean surfaces with oxidative stress. By learning how the chemical structure relates to reactivity, both students and experienced chemists can spot new uses for classic molecules like 3,3’-Dithiodipropionic acid.
Challenges and Future Solutions
Some hurdles remain. Sourcing high-purity samples can be costly, and the molecule’s reactivity sometimes complicates storage for long-term studies. Firms seeking to innovate with green chemistry principles face the challenge of minimizing waste and hazardous byproducts. Shifting towards solvent-free synthesis or recycling processes can help reduce environmental impact.
Education forms another key plank. Better resources for students and professionals alike would sharpen awareness of the molecule’s potential and the correct practices for handling it. New partnerships between university labs and manufacturing industries could pave the way for scaled-up, eco-friendly production. As chemists look to the future, understanding – at the level of every atom and bond – remains the first step toward safer, smarter, and more sustainable practice.
Understanding What We’re Working With
Talking about chemical safety often leads to confusing territory. Many people ask if 3,3'-Dithiodipropionic Acid is dangerous, but answering that means digging into how it works, what scientists know, and where it shows up.
This compound isn’t a household name. You probably won’t spot it under your sink, but labs and industry settings use it in research and manufacturing. The structure contains sulfur and carboxylic acid groups, and chemists sometimes call it DTDP acid. Beyond the lab, it hasn’t made a big splash, which can make reliable health and safety info harder to find.
Possible Risks in Handling
Most data puts 3,3'-Dithiodipropionic Acid in the “irritant” category. Direct skin or eye contact tends to sting or redden skin. Scientists handling it in pure or concentrated form wear gloves, goggles, and lab coats for good reason. It’s not something you want near your face or hands for long. Breathing in its dust over time could cause throat or lung irritation, just like with many finely powdered chemicals.
Large-scale toxicity studies – the kind that look for cancer risk, long-term genetic damage, or reproductive hazards – haven’t flagged this compound as highly dangerous. The European Chemicals Agency classifies it as irritating but stops short of tougher labels like “toxic” or “carcinogenic.” Accidents can still happen. I’ve seen chemistry students splash acids without thinking, only to regret it a few hours later when irritation creeps up. Protective steps are not something anyone working around chemicals skips.
Environmental Concerns
Researchers track where chemicals go once spilled or washed down the drain. 3,3'-Dithiodipropionic Acid doesn’t seem to stick around or build up in food chains. Early studies show bacteria can break it down in wastewater. It doesn’t carry the “persistent organic pollutant” tag that worries environmentalists. Still, care around drainage and waste is smart. Treating chemical waste properly is standard in most labs and factories, and environmental regulators watch for improper discharge. I’ve seen environmental reviews where the main focus landed not on this compound itself, but on keeping waste processes up to code.
Toxicity and Exposure — What We Actually Know
Medical reports connect high levels of exposure (through accidents or spills) to mild symptoms like headache or lightheadedness. There aren’t major public records showing people hospitalized from this compound. It also doesn’t show up in the lists of chemicals with long-term neurological or organ damage affects, as seen with mercuric compounds, lead, or even common pesticides.
Exposure in ordinary life remains extremely unlikely. For those who brew it up in a lab, the same rules always apply: know your procedures, respect the material, and don’t work alone if there’s any hint of danger. Official chemical safety data sheets (SDS) offer all the right advice. Gloves, splash-proof goggles, and good ventilation keep nearly every risk in check. Spills get handled fast, and large exposures rarely happen where trained chemists run the show. In my experience on the lab floor, simple attention to safety gear and chemical hygiene heads off almost all problems.
Solutions for Safer Use
Basic safety steps beat complicated solutions almost every time. Good labeling, clear training materials, and up-to-date waste handling reduce risk. For new chemicals, read the SDS, ask the safety officer for help, and never work distracted. Respect for chemicals takes root from the first chemistry class and grows stronger with every safe day in the lab. Companies and universities already demand these routines, and safety inspections back them up. With efforts focused on solid procedures, 3,3'-Dithiodipropionic Acid stays low on the hazard list for anyone using it responsibly.
3,3'-Dithiodipropionic acid often shows up in labs and chemical stores as a reagent, sometimes in research focused on antioxidants or as a cross-linking agent. Folks who deal with it know the usual: it’s a white to off-white crystalline powder, not terribly famous outside of scientific circles. Yet, just like any specialty chemical, this compound, if left to its own devices, doesn’t always play nice.
Why Storage Isn't Just About Space
Picture this: the label says “store in a cool, dry place,” and it’s tempting to just park the jar next to other powders. Not so fast. Experience with similar thio compounds shows you can’t always tell by looks when something might react with humidity, light, or airborne contaminants. Given its disulfide bonds, extra moisture spells trouble. Over time, humidity can lead to clumping, loss of purity, or even alteration into something you didn’t bargain for.
The Real Deal With Containers
You can’t just throw this acid into any container and hope for the best. Polyethylene bottles, glass jars with tight screw caps, or sealed aluminum pouches offer a better bet because air and water won’t sneak in so easily. The reasons matter: air-tight seals keep out oxygen and water vapor, both of which can start slow chemical changes, making your acid unreliable in your next experiment.
Why Temperature Matters
Chemical folklore says “Room temperature, away from direct sunlight.” Science agrees—higher temperatures speed up reactions you might not see until later. Storing these bottles far from heat vents or sunlit windows helps, since even a mild temperature spike can ruin a batch in weeks, not years. If climate control feels like a hassle, weigh that against ruining a month’s work because of degraded chemical stock.
Security and Labeling: Not Just for Show
Not every chemical thief wears a mask. Confusion, “oops moments,” and tired hands cause more trouble in the lab than any hacker. Labels go a long way—write dates, keep MSDS sheets handy, and resist the urge to reuse old labels or containers. It takes one wrong scoop from a mystery jar to ruin a multi-step synthesis, probably contaminating neighboring samples for good measure.
Mistakes Happen. How to Clean Up
Should you spill dithiodipropionic acid, grab gloves and sweep instead of vacuuming or brushing with bare hands. Spills remind everyone why proper storage pays off. Not all acids burn or smoke, but this one, like most organics, won’t make you healthier if you breathe the dust or absorb it through cracked skin.
Learning From the Pros: Real Lab Stories
Talking to career chemists shows shortcuts rarely work. An old trick borrowed from protein labs—store sensitive powders with a bag of desiccant, refreshing it every month. Dry boxes and silica gel packs offer cheap insurance. Even a zip-top bag adds another layer of security. It’s not about expensive gear; it’s about habits that keep your stuff clean and effective.
Keeping Chemicals Useful and Safe
A careless storage plan can leave you with mystery lumps and wasted money. With a climate-stable spot, dry containers, and clear labels, 3,3'-dithiodipropionic acid will be just as good in a year as the day you popped open the jar. That’s not just best practice—it saves frustration and lets research move forward, one reliable experiment at a time.
Making Stronger Polymers That Last
Walk into a plastics research lab and you might spot chemists adding 3,3'-Dithiodipropionic Acid to their formulas. This compound brings sulfur-rich bridges into polymer chains, which give everyday plastics more resistance against breaking down. Think about how a plastic bottle is always fighting off sunlight, heat, and even corrosion. Sulfur bonds create cross-links in the material, strengthening it and helping it stand up against rough conditions. These robust materials find their way into car parts, durable packaging, and even medical devices that come into contact with harsh chemicals.
Essential in Drug Delivery and Biomedical Devices
Biomedical engineers use this compound for its unique ability to form and break bonds under gentle conditions. For example, drug delivery capsules use tailorable coatings made from polymers linked together by this acid. In a healthy gut or bloodstream, these coatings stay locked, holding in the medicine. Once they reach a targeted cell or tissue rich in certain chemical triggers—like glutathione inside cells—these sulfur bridges snap open, releasing the drug at just the right spot. This selective release has made the acid popular in designing advanced cancer treatments that minimize damage to healthy tissue.
Helping with Antioxidants and Preservatives
Food scientists and nutritionists have turned to 3,3'-Dithiodipropionic Acid for its abilities as an antioxidant precursor. The compound, when metabolized, can help boost glutathione—a major antioxidant inside the body. Some animal studies show that adding small amounts of it to feed can raise antioxidant levels, helping livestock deal with stress. In other sectors, chemical manufacturers use it in phenolic resin production, making tough composites for brake pads and insulation that resist oxidation much longer than older versions.
Building Better Sensors and Electronics
Next time you swipe your ID badge or watch a sensor-triggered door open, think of the tiny molecules working behind the scenes. Biosensor designers use 3,3'-Dithiodipropionic Acid to immobilize proteins or antibodies on gold surfaces. The sulfur groups in the acid stick firmly to the gold, acting as a sturdy anchor for sensitive detector layers. These attachments are stable and allow for highly specific identification of chemicals, toxins, or even viruses. By controlling how molecules stick together, labs can craft sensors used in hospitals, food safety checks, and pollution monitoring.
Green Chemistry and Waste Cleanup
Over the years, chemists working on sustainable solutions looked to 3,3'-Dithiodipropionic Acid for its role in biodegradable plastics. The biomarkers from its degradation help monitor how fast plastics break down in soil or water. Wastewater treatment researchers use it as a sulfur source in experimental systems aimed at cleaning up heavy metals like mercury or lead, since sulfur bonds grab these metals tightly, assisting in removal from water supplies.
Moving Forward with Smarter Materials
Researchers keep hunting for new uses for this compound, especially as industries push for greener chemicals and smarter, targeted therapies. Controlled-release pharmaceuticals, stronger composite materials, and more sensitive environmental sensors all depend on the chemistry this acid brings. By supporting further funding, universities and businesses can bring even better products and safer solutions to the public.
| Names | |
| Preferred IUPAC name | 3,3'-Disulfanediyldipropanoic acid |
| Other names |
bis(3-carboxypropyl) disulfide
DTDP thiodipropionic acid 3,3’-dithiobispropionic acid 3,3’-Thiodipropionic acid |
| Pronunciation | /ˌθriː θriː ˌdɪθaɪ.oʊ daɪ proʊˈpiːɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 1119-62-6 |
| Beilstein Reference | 1208720 |
| ChEBI | CHEBI:37144 |
| ChEMBL | CHEMBL97206 |
| ChemSpider | 12910 |
| DrugBank | DB04125 |
| ECHA InfoCard | ECHA InfoCard: 100.007.701 |
| EC Number | 211-746-3 |
| Gmelin Reference | 6077 |
| KEGG | C11104 |
| MeSH | D006172 |
| PubChem CID | 8218 |
| RTECS number | TZ7875000 |
| UNII | 5B8986L4EI |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID8046421 |
| Properties | |
| Chemical formula | C6H10O4S2 |
| Molar mass | 210.27 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.46 g/cm3 |
| Solubility in water | Soluble in water |
| log P | -2.0 |
| Vapor pressure | 0.01 mmHg (20°C) |
| Acidity (pKa) | 4.3 |
| Basicity (pKb) | pKb: 5.05 |
| Magnetic susceptibility (χ) | -67.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.570 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.21 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 180.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -474.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1173.9 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P260, P264, P270, P273, P301+P312, P330, P501 |
| Flash point | 204 °C |
| Lethal dose or concentration | LD50 oral (rat): 2,200 mg/kg |
| LD50 (median dose) | LD50: Oral, Rat = 3200 mg/kg |
| NIOSH | Onion oil |
| PEL (Permissible) | Not established |
| REL (Recommended) | REL: 5 mg/m³ |
| IDLH (Immediate danger) | Not Established |
| Related compounds | |
| Related compounds |
Thiodipropionic acid
3-Mercaptopropionic acid Disulfide compounds Cystamine Dimercaptosuccinic acid |
