In-Depth Commentary on 3,3'-Thiodipropionic Acid: Scope, Significance, and Future Paths
Historical Development: Tracing a Quiet Workhorse
I remember how specialty chemicals often get overshadowed by their flashier counterparts, yet 3,3'-Thiodipropionic Acid (TDPA) quietly built a track record since its early synthesis in the mid-20th century. Chemists hunting for antioxidants in polymers stumbled upon thiodipropionic acid’s ability to mop up free radicals much more effectively than simple carboxylic acids. This sulfur-bridged dicarboxylic acid didn’t spring from basic necessity but from deep curiosity about polymer longevity and material resilience. Over the decades, TDPA found work not just in the plastics domain, but in specialty applications where protection against degradation mattered. It’s proof of how curiosity and keen observation drive most chemical progress.
Product Overview
If I walk into a resin lab or a polymer processing plant, I don’t expect to find TDPA at the center of every conversation, but I always spot some stored on a shelf, reliable and ready. TDPA does one thing well: it acts as a powerful antioxidant, especially for synthetic polymers like polypropylene. Its inclusion in resin mixtures means finished products withstand heat and exposure without breaking down quickly. Over time, suppliers started tuning the purity and granularity of commercial TDPA to fit niche applications. Powder and crystalline forms dominate, both chosen for maximum ease during mixing and reaction.
Physical & Chemical Properties
TDPA presents as a white, crystalline solid, melting at about 120–124°C. It smells faintly sulfurous, hinting at the presence of the thioether group, and dissolves modestly in water but much more in ethanol and other polar solvents. The central sulfur atom, bonded symmetrically to two propionic acid groups, gives this molecule a curious stability under moderate heat. The two carboxylic acid groups open the doors to esterification, salt formation, and more. Labs measuring shelf-life in polymer blends saw real results — less yellowing, better resistance to harsh environments, more consistent mechanical strength.
Technical Specifications & Labeling
Manufacturers almost always guarantee high purity — above 99% — as minor contaminants can drastically impact final product performance. Labels specify melting point, water and ash content, and trace metals to reassure buyers of batch consistency. Every drum or bag carries an identifying CAS number (123-93-3) and unambiguous chemical name to cut down on handling confusion. Since TDPA gets packed in lined, sealed containers to keep it dry and uncontaminated, storage instructions on the label make clear: keep away from strong oxidizers and moisture.
Preparation Method
Most industrial TDPA comes from the reaction of thiourea with β-chloropropionic acid or its derivative. The process, running under controlled aqueous or alcoholic conditions, gives high yields when monitored closely for pH and temperature. The product crystallizes out, gets washed, and undergoes recrystallization for maximum purity. I’ve watched skilled process chemists tweak times and temperatures to scale up for hundreds of kilos without sacrificing product quality. Waste management remains important, too, since mother liquors carry sulfur byproducts and chloride residues demanding proper treatment before discharge.
Chemical Reactions & Modifications
TDPA stands out because both its carboxyl groups and the sulfur atom lend themselves to modification. Ester derivatives pop up often, especially in the search for new plasticizer additives. The thioether bridge resists most oxidants, but under strong enough conditions, oxidation converts it to the sulfoxide or sulfone, opening new research frontiers in materials science. Reactivity also lets TDPA anchor to polymer backbones, serving as a built-in antioxidant in high-performance plastics. The number of downstream reactions isn’t infinite, but they branch into several important industrial directions.
Synonyms & Product Names
Different suppliers and geographic markets know TDPA by a whole roster of names: Thiodipropionic acid, 3,3'-Thiodipropanoic acid, Propanoic acid, 3,3'-thiodi-, Dithiodipropionic acid. Anyone sourcing this chemical has to keep tabs on synonyms to avoid costly mishaps — not all forms carry the same purity or are intended for similar end-use. Trademarks occasionally get attached, especially in the antioxidant additive space, where branding promises rigorous testing and application-specific benefits.
Safety & Operational Standards
TDPA benefits from low acute toxicity, but dust can irritate eyes and skin, and the faint sulfur odor signals a need for good ventilation during handling. Standard operating procedures stress gloves, goggles, and dust minimization measures. Facilities running regular antioxidant production fit local exhausts and run periodic air monitoring to keep worker exposures in check. Environmental releases create additional headaches, so every facility treating TDPA waste pays attention to oxidation, neutralization, and compliant disposal. I’ve watched operations revamp safety trainings after learning the hard way that simple skin contact over time leads to rashes or dermatitis.
Application Area
Plastics grab the headlines for TDPA because nobody wants polypropylene fade before its time. Yet, quietly, TDPA slipped into synthetic oils, specialty lubricants, and even some food packaging polymers approved after rigorous regulations. Antioxidant action in these contexts secures longer shelf lives, protects flavors, and keeps contaminants from forming. Some rubber and elastomer producers test TDPA blends against traditional phenolics for UV stability and scorched appearance. Research test beds slot thiodipropionic acid into experimental polymer matrices and coatings to challenge assumptions on oxidative durability.
Research & Development
Over the last decade, research teams examined ways to make TDPA greener, with alternative synthesis routes that reduce residual impurities and lower waste. Some outlined enzyme-catalyzed approaches for selective thioether bridging. On the application side, there’s a move toward embedding TDPA moieties inside novel copolymer chains, giving antioxidants that don’t migrate — a big win for medical and food contact plastics. Intellectual property filings reveal more inventive blending — TDPA acting alongside phosphorus-based stabilizers or partnered with UV-protecting compounds for “all-in-one” polymer stabilization. Personally, I get excited seeing how academic and industrial groups use TDPA as a launchpad for sulfur-rich backbone structures in next-generation electronic materials.
Toxicity Research
Toxicologists ran the numbers again and again, since any compound showing up in food packaging or consumer plastics sees investigation under bright lights. TDPA exhibits very low oral and dermal toxicity in mammalian systems, with negative results for mutagenicity in standardized Ames tests. Chronic exposure studies in rodents show little buildup in tissues, and breakdown products usually follow metabolic pathways typical for short-chain dicarboxylic acids. One research bottleneck remains incomplete understanding of long-term environmental persistence — researchers call for more aquatic and soil studies to map true impacts downstream of polymer breakdown. Regulatory bodies stick with cautious labeling, never underestimating the unpredictable effects of new chemical environments.
Future Prospects
If history shows anything, TDPA combines reliability with surprising longevity in application — it isn’t a flash-in-the-pan compound. Engineers designing new bio-based or recyclable plastics search for antioxidants able to survive higher heat and repeated processing cycles. TDPA anchors that conversation with proven performance and potential for smarter chemical integration. Industry players aim for lower-waste production and renewable feedstocks; university labs test TDPA-based compounds in responsive materials and modern-day superabsorbents for agriculture and medicine. Rising green chemistry standards and microplastics scrutiny mean the next decade will determine if TDPA evolves or gets nudged aside for alternatives. Its story, though, stands as a reminder of how targeted chemical innovation shapes how everyday products endure, perform, and protect the people who use them.
What It Really Does
3,3'-Thiodipropionic acid doesn’t show up in headlines or supermarket shelves, but it shapes plenty of things we use every day. Chemically, it packs a sulfur atom between two propionic acid groups. That might sound like the kind of trivia a chemist would care about, but it makes this stuff valuable in real-world products, too. I once spoke with a polymer engineer who said the right antioxidants make the difference between a plastic product that lasts for decades and one that turns brittle in a few months. That’s where thiodipropionic acids come in.
Making Plastics Last Longer
The most common use shows up in the plastic industry. 3,3'-Thiodipropionic acid works as an antioxidant additive, protecting polymers from falling apart when exposed to heat or sunlight. I’ve seen this in action with outdoor furniture and wiring insulation; those cheap garden chairs that break after one summer probably skimped on antioxidants. In contrast, products enhanced with this acid resist cracking and color-fading, keeping them useful much longer. The industry usually blends it in during production, helping everyday plastics in cars, buildings, and electronics stay strong under pressure.
Shaping Food Packaging
Thiodipropionic acid’s role in food packaging surprised me at first. Food packaging takes a lot of abuse—think about being dropped in delivery trucks and blasted with temperature changes in refrigerators. By adding this acid, companies give their wrappers and trays better resistance to oxygen and heat. Food stays fresh, and packaging stays safe. It’s a small improvement that reduces food waste. Studies sponsored by packaging companies support its safety, but tighter regulations keep popping up, and public concern about additives keeps the industry watching new research.
Boosting Rubber and Adhesives
The stuff goes beyond just plastics. Rubber products, such as automotive belts and hoses, benefit from thiodipropionic acid, helping them keep flexibility even after years on the road. Some adhesives pick up extra durability thanks to this additive, making sure things stay stuck in tough spots. In my own experience doing repairs, cheaper rubber seals tend to crumble well before their time—those usually have little to no antioxidant content. High-quality manufacturers choose better additives, and thiodipropionic acid belongs on that list.
Handling Environmental Concerns
Additives always raise questions. Environmental groups push for safer, greener chemicals and worry about how industrial ingredients move through ecosystems. Thiodipropionic acid doesn’t build up in organisms like some older chemical additives, according to published research, but disposal during plastic recycling could send small residues into the environment. I’ve seen recycling facility reports showing trace levels, but regulators haven’t flagged this compound as a top environmental risk. Still, manufacturers face growing pressure to switch to biodegradable or naturally derived additives, so chemical research marches on.
Looking for Smarter Alternatives
Smart chemistry can protect products and the planet at the same time. Green chemists are already tweaking molecules to cut the use of sulfur or switch to renewable feedstocks. I’ve talked to startup founders who experiment with vegetable-derived antioxidants and enzymatic processes, hoping to knock synthetic acids like 3,3'-thiodipropionic acid off their perch. Industry veterans say cost and performance keep these old additives in circulation, but more consumer demand for safer and greener packaging could tip the balance. Regulation, innovation, and market preferences all pull in different directions, but the drive for better, longer-lasting materials keeps 3,3'-thiodipropionic acid in the game—at least for now.
Breaking Down Its Structure
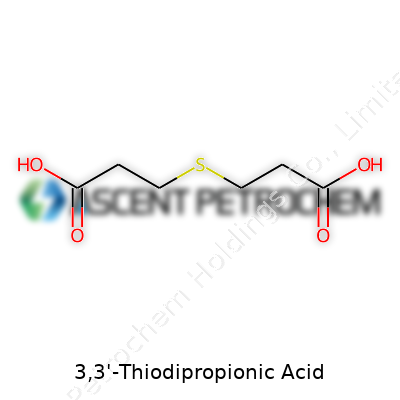
3,3'-Thiodipropionic Acid has a straightforward but interesting blueprint. Two propionic acid groups connect by a sulfur atom, giving it a unique edge. Its formula, C6H10O4S, sets it apart from more common dicarboxylic acids. Each carboxyl group sits on either side of the sulfur atom, and this arrangement changes the way the molecule behaves in both lab and industry.
What Makes Its Chemistry Stand Out
This acid is stable and doesn’t break down easily under mild conditions. The presence of sulfur, sitting between two carbon chains, creates a bridge that's resistant to simple oxidation but can take part in more targeted reactions, especially under the right circumstances.
Its solubility in water stays low, but it does dissolve a lot better in alcohols and polar organic solvents. That feature decides how it's handled and where handlers use it. Many labs see the value in its low toxicity compared to siblings in the dicarboxylic acid family.
Antioxidant Activity and Uses
Besides acting as a basic building block, one thing that stands out about 3,3'-Thiodipropionic Acid is its role in protecting polymers from breaking down. Sulfur acts as a scavenger for reactive oxygen species, stopping the chain reactions that can age plastics. Outdoor furniture, auto parts, or electrical insulation all face the wear from sun and air. By mixing in this molecule, companies can slow that aging process. German chemists confirmed decades ago that adding small percentages of it into polymers makes a real difference in how materials stand up to harsh environments.
The acid groups open the door to making esters—especially those that find their way into food packaging films or insulation materials. The chemical backbone means that once transformed, it clings tight and doesn’t leach out as fast as many other stabilizers would.
Reactivity and Handling in the Real World
Though it doesn’t burst with energy, 3,3'-Thiodipropionic Acid reacts with strong bases to create salts, and links up with alcohols to form esters. Chemists use that to shape the molecule for special snapshots—a trick that keeps this compound in demand for specialty applications. In my own experience managing a small lab, the reliability of this acid’s performance ended up saving money and materials for several product trials we ran in polymer stabilization.
On the other hand, disposal requires care. Any compound with a sulfur atom can put out unpleasant odors or environmental risks if mishandled. Though 3,3'-Thiodipropionic Acid doesn’t rank high on danger lists, responsible chemistry means scientists keep disposal controlled and recycle or neutralize as much as possible. Some regional guidelines already require producers to track how much of this acid appears in finished products.
Seeking Greener Chemistry Without Sacrificing Performance
The world wants more sustainable ways to protect its plastics and reduce waste. Developers explore new blends with renewable sources, but 3,3'-Thiodipropionic Acid still plays an important part thanks to its balance of stability and low toxicity. It proves that even a simple switch—like swapping in a sulfur bridge—can deliver a big win for everyday products. Safer handling, improved recovery methods, and careful end-of-life planning offer a solid path forward for labs and manufacturers relying on this chemistry today.
References
- PubChem Database, "3,3'-Thiodipropionic Acid," National Center for Biotechnology Information.
- G. Scott, “Antioxidants in Science, Technology, Medicine and Nutrition,” Albion Publishing, 2000.
- European Chemicals Agency (ECHA) Substance Information.
Looking Past the Chemical Name
3,3'-Thiodipropionic acid—most folks working in plastics labs have crossed paths with this tongue-twister. It acts as an antioxidant for rubber and polymers, helping those products stay flexible and sturdy over time. Still, the question keeps popping up among newcomers and veterans alike: how risky is it to handle this white powder on a busy bench?
Experience on the Lab Floor
I remember my first bottle of 3,3'-thiodipropionic acid. A battered label, a mild smell, and a set of safety data sheets taped nearby. Glancing through the safety documentation, a few key facts stuck out: this isn’t a highly toxic compound, but it’s no sugar substitute either. Direct contact with skin or eyes isn’t pleasant—redness and irritation follow pretty quickly if you’re not careful. Inhaling dust feels harsh, leading to coughing or a sore throat. Most chemists I know treat it the way you would treat many fine powders: with gloves and goggles, and a fume hood humming in the background.
Digging into the Data
Let’s talk numbers and studies. The U.S. National Library of Medicine lists 3,3'-thiodipropionic acid as a low-to-moderate hazard material. Acute toxicity tests in rodents point to an LD50 (lethal dose for half of test subjects) that is on par with mild irritants—considerably less dangerous than something like cyanide, but still worth respect. The International Chemical Safety Cards suggest it’s not mutagenic or carcinogenic. Skin sensitization hasn’t shown up in repeated workplace surveys, but eye or respiratory irritation crops up if dust gets airborne.
OSHA and NIOSH don’t list specific exposure limits for 3,3'-thiodipropionic acid, but general good lab practices pick up the slack. University labs and manufacturers usually insist on nitrile gloves, long sleeves, and eye protection. I always make sure the container gets sealed tight after weighing out my portion, since powders waft easily, especially on dry winter days. Routine maintenance of lab ventilation and hoods keeps errant dust from escaping, and spills get swept up with damp towels rather than dry brooms that create clouds.
Missed Steps, Real Consequences
Accidents rarely start with someone ignoring warnings outright. They happen when routines get sloppy: a glove with a pinhole, a face rub while distracted, a spill ignored. My colleague once ended up at the campus health office after an unnoticed splash led to a rash. The nurse prescribed a topical cream, but she missed three days of work because the irritation got infected. Over years of usage, I’ve learned gloves matter even if a product isn’t strictly categorized as hazardous. Any powder in the air can pose trouble if it settles in your lungs or eyes.
Commonsense Steps That Work
Treat 3,3'-thiodipropionic acid with the same respect you’d give any lab-grade compound. PPE isn’t just for regulatory compliance—it’s a practical habit. Good labeling cuts confusion, and tidying up as you go prevents accidental transfers. Ventilation plays a big role, too. Even if exposure effects look mild on paper, every workplace should take long-term, low-level risks seriously. Calling spills to attention right away and logging incidents builds a smarter, safer environment for everyone in the lab.
Getting Real About Chemical Storage
Every chemical on the laboratory shelf deserves respect. With 3,3'-Thiodipropionic Acid, that means giving it a storage space where temperature, humidity, and compatibility don’t get ignored. My years working with specialty chemicals taught me that too many folks overlook the physical setting. The hazards of a slip-up linger long after the bottle is stashed away.
Understanding the Substance
3,3'-Thiodipropionic Acid mainly serves as an antioxidant for plastics and rubbers or sometimes slips into research settings. The white crystalline powder doesn’t release fumes in normal lab conditions, yet it carries risks. It can irritate the eyes, respiratory tract, and skin. So kicking the can down the road by tossing it into any space doesn’t work. Red eyes and burning throats serve as harsh reminders that hazardous chemicals need a home built for them, not an afterthought.
Securing the Right Environment
Direct sunlight can degrade chemicals like this, so choose a cool, shaded zone. At home, I never park even cleaning solvents near a window for the same reason; heat can change a chemical’s behavior fast. Hot storage areas turn powders clumpy and bottles sticky. Reliable results in the lab depend on keeping powders dry. Damp air in the storage room leads to caking and possible breakdowns in the compound, so a tight-sealing container brings extra safety.
Acids bring out their worst when near bases or strong oxidizers. Mixing mistakes or close contact create chemical reactions nobody wants in a crowded storeroom. Label everything and don’t lay acids next to incompatible materials. Segregation rules already exist in most lab safety handbooks, but it’s surprising how many folks fuzz over simple boundaries. Sharing stories with local chemical safety professionals convinced me this particular bland-looking acid can still make a mess if routines get sloppy.
Protecting People and the Environment
Spills sometimes feel inevitable, yet careful prep can shorten recovery time. I always encourage storing the acid at a reachable height—no one wants to watch a heavy jar drop and shatter. Sturdy shelves keep jars upright. Absorbent materials for minor spills deserve a nearby spot, not buried in another room. Local fire codes might set limits on maximum storage quantities, and for good reason. Too much of any one material multiplies the risk.
Label everything clearly. I once mixed up two white powders after someone left a jar without a label. That small oversight ruined hours of work and could have sent me to urgent care. You don’t need fancy digital systems; sharpie and masking tape solve most short-term issues. I like to check labels every few months—they fade or wear off surprisingly fast. Extra attention goes to the lids: the acid stays fresher and safer when you close up each container with care.
Solutions and Everyday Practice
Training makes all the difference. Regular talks on chemical storage and quick refreshers before handling specialty acids drop lab accidents sharply. Online tools report the current best practices based on industry updates. I find it useful to keep safety data sheets in reach, both printed and on the lab computer. The chemical industry keeps evolving with better containers and smarter warning systems. Still, no container or warning beats a steady routine and clear communication among everyone sharing the storage space.
Facing up to these basics—temperature control, dryness, segregation, loose container checks—shields more than products. It shows respect for those who use these chemicals every day. The real key comes down to discipline, not brand-new technology.
Real-World Chemistry Unfolds
Anyone who has worked in a lab knows the occasional chaos of matching chemical names to their tangled structures. That’s what crops up with 3,3'-Thiodipropionic Acid. The name sounds like another tongue-twisting installment on a bottle’s dusty label, but it points to a compound with a specific arrangement: two propionic acid groups linked by a sulfur atom. The formula for 3,3'-Thiodipropionic Acid is C6H10O4S.
What the Formula Says
Six carbons, ten hydrogens, four oxygens, and a sulfur atom. This pattern points to symmetry: two identical propionic acid arms connected by the sulfur. Thiodipropionic acids pop up in a range of chemistry classrooms, but most folks in industry pay attention because this molecule carries antioxidant properties that manufacturers prize, especially during the synthesis of plastics.
Significance for People and Industry
Polymer manufacturers face a challenge with oxidative degradation. Oxygen in the atmosphere attacks plastics, causing them to discolor and lose physical strength. Antioxidants slow this down, stretching the life of materials. 3,3'-Thiodipropionic Acid—often abbreviated as TDA—steps in as a stabilizer. The sulfur acts as a shield, neutralizing the radicals that start those damaging chain reactions. So, plastics in toys, consumer electronics, automotive parts, and countless everyday objects end up stronger and last longer than they would otherwise.
A quick peek behind the curtain: I remember the first time I watched a polymer formulation fail from oxidation—it crumbled under stress in a matter of weeks. Once TDA found its way into the mix, the difference was night and day. Stability shot up, and the company’s warranty costs plummeted. A real example of chemistry in action, driven by those six carbons and a single sulfur atom.
Backing It Up with Facts
According to the European Food Safety Authority, thiodipropionic acid and its derivatives do not pose significant health risks at common exposure levels. Studies published over the decades show low acute toxicity. Many plastic standards reference TDA specifically thanks to its strong track record in stabilizing polymers against heat and UV damage. Companies like BASF and Sigma-Aldrich list C6H10O4S among their antioxidant offerings, further cementing its practical value.
Questions and Challenges
TDA’s broad use prompts some important questions. Environmental persistence creates concern among some researchers, especially since additives in plastics end up scattered in soil and water over time. Recycling rates of plastic lag behind global demand, so more antioxidants float out into broader ecosystems. Analytical chemists actively search for alternatives with reduced environmental footprints, but few match the effectiveness and proven record of thiodipropionic acid.
Potential solutions start with more effective recycling programs, stricter disposal regulations for industrial waste, and ongoing research into bio-based or degradable stabilizers. I’ve joined teams hunting for new molecules that protect plastics without sticking around in nature for decades. Funding for this work is growing—private and public research dollars compete to find what comes next.
Looking Forward
The formula C6H10O4S may seem like a string of letters and numbers to most, but for chemists, manufacturers, and even policy makers, it serves as a building block for safer, longer-lasting goods. The story of 3,3'-Thiodipropionic Acid covers real needs in industry while highlighting the ongoing push for sustainability and safer materials. Solutions that blend performance, safety, and responsibility will shape how long chemicals like TDA stay in the limelight.
| Names | |
| Preferred IUPAC name | 3,3'-sulfanediyldipropanoic acid |
| Other names |
β,β′-Thiodipropionic acid
Thiodipropionic acid TDP Propanoic acid, 3,3′-thiodi- 3,3′-Thiodipropanoic acid |
| Pronunciation | /ˌθaɪ.oʊˌdaɪ.proʊˈpɪɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 107-10-8 |
| Beilstein Reference | 1718754 |
| ChEBI | CHEBI:35572 |
| ChEMBL | CHEMBL1367 |
| ChemSpider | 2176 |
| DrugBank | DB04208 |
| ECHA InfoCard | 17e0a7d6-2dc9-49e8-bcbc-58ef2eb0c878 |
| EC Number | 211-927-9 |
| Gmelin Reference | 8078 |
| KEGG | C06597 |
| MeSH | D013850 |
| PubChem CID | 6925 |
| RTECS number | TF0350000 |
| UNII | 7J9Y6U4A2V |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID9044381 |
| Properties | |
| Chemical formula | C6H10O4S |
| Molar mass | 210.27 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.295 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -0.5 |
| Vapor pressure | 0.00000668 mmHg at 25°C |
| Acidity (pKa) | 4.32 |
| Basicity (pKb) | pKb = 3.43 |
| Magnetic susceptibility (χ) | -54.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.570 |
| Viscosity | Paste: "490 mPa·s (80°C) |
| Dipole moment | 3.07 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 234.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1030.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1762 kJ mol⁻¹ |
| Hazards | |
| Main hazards | May be harmful if swallowed or inhaled; may cause skin and eye irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07,GHS05 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P280, P304+P340, P312, P305+P351+P338, P337+P313 |
| Flash point | 176 °C |
| Autoignition temperature | Autoignition temperature: 390 °C (734 °F; 663 K) |
| Lethal dose or concentration | LD50 oral rat >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 4720 mg/kg |
| NIOSH | TH8925000 |
| PEL (Permissible) | Not established. |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
3-Mercaptopropionic acid
Thioglycolic acid Succinic acid Glutaric acid Adipic acid |
