3-Mercaptopropionic Acid: A Deep Dive into a Crucial Chemical
Historical Development
Long before today’s precision chemistry took hold, basic building blocks like 3-mercaptopropionic acid (3-MPA) found their way into labs through the persistent work of experimental chemists. The compound’s history dates back to the rise of organosulfur chemistry during the mid-20th century, when researchers explored sulfur’s unique reactivity. Early synthesis efforts revealed that adding a thiol group to short-chain carboxylic acids could create substances with both demanding odors and invaluable chemical potential. I’ve seen researchers cite the initial trial-and-error years, including process optimizations through distillation and selective reduction, as a reminder that patience builds the backbone of chemical progress. Today’s large-scale production owes a debt to those early organic chemists who stubbornly refined their isolation skills.
Product Overview
Any well-equipped laboratory or manufacturing plant handling specialty chemicals will come across bottles labeled ‘3-Mercaptopropionic Acid’, a clear, colorless to pale yellow liquid with a powerful mercaptan scent. Chemists use this acid to introduce thiol groups into organic frameworks, opening doors to a host of specialty syntheses in pharmaceuticals, coatings, and catalysts. The molecule packs both sulfur and carboxyl functionalities in just three carbon atoms, which translates to a strong affinity for metal surfaces and cross-linking reactions. Researchers value its low molecular weight—just 106.15 grams per mole—for ease of handling and predictable stoichiometry. Packaging tends to use amber-glass containers fitted with secure lids to protect the pungent contents from light and air.
Physical & Chemical Properties
Anyone who’s poured a drop of 3-Mercaptopropionic Acid knows its sharp, sulfurous smell hits you instantly. It boils near 93°C at 2 mmHg, melts below -18°C, and dissolves readily in water and organic solvents. The presence of both thiol and carboxyl groups produces significant reactivity: the thiol moiety interacts with electrophiles or oxidizes to disulfides, while the acid group can form salts or esters. Its density (around 1.23 g/cm³) and viscosity prove manageable for pipetting or bulk transfer, aspects any bench chemist appreciates when scaling up processes. Solubility lets it act as an effective linker in bioconjugation work, where reliability in aqueous and organic media matters. While handling this compound, I’ve found even trace impurities—especially oxidized disulfides—can alter outcomes, so attention to purity takes the front seat.
Technical Specifications & Labeling
Labeling provides a lifeline for safety and consistency: 3-Mercaptopropionic Acid typically carries a purity above 98%, and technical sheets list moisture content, heavy metal thresholds, pH in solution, and presence of residual solvents. Container labels warn of pungency and skin sensitivity, plus guidance for personal protective equipment. Documentation often includes the CAS number (107-96-0), molecular formula (C3H6O2S), and key hazard pictograms. Producers outline shelf life and storage temperatures, stressing cool, well-ventilated areas away from oxidizers. In every reputable supplier’s literature, I’ve found full traceability—batch number, production date, QC log—helping users ensure their material meets stringent project requirements.
Preparation Method
Synthesis follows a direct and reliable route: the nucleophilic addition of hydrogen sulfide to acrylic acid remains a favored approach, either under acidic catalysis or with gaseous H2S bubbled through an acrylate solution. Temperature, pH, and strict exclusion of oxygen govern the yield and prevent unwanted oxidation of the thiol. Over time, refinements replaced hazardous processes with safer alternatives, such as sodium sulfide reduction in water, which I know has lowered operational risks. Scale-up hinges on efficient purification—vacuum distillation or extraction—because the pungent smell can linger long after the main product forms. Factories investing in robust fume management get my respect; they turn what could be a stubborn occupational hazard into a safe, dry powder or liquid for commercial markets.
Chemical Reactions & Modifications
The dual nature of 3-Mercaptopropionic Acid gives chemists plenty to work with. The thiol group reacts with alkyl halides to yield thioethers, couples with maleimides in protein conjugations, or oxidizes into disulfides in air or with mild oxidizers. The carboxyl group serves as a site for esterification, amide formation, or salt generation. In practical lab work, I’ve witnessed how crosslinking reactions in polymer chemistry use the compound to introduce flexibility or adhesive properties. Researchers sometimes modify 3-MPA by protecting the thiol during multistep syntheses or by derivatizing the acid group to improve solubility. Exploring reactivity maps highlights that even simple compounds, when handled with creativity, become useful for diverse molecular modifications—from drug analogues to cutting-edge nanomaterials.
Synonyms & Product Names
Many chemical catalogs list alternative names: β-Mercaptopropionic Acid, 3-MPA, MPA, or Thiolactic Acid. Product references might use the EINECS number (203-537-0) or other commercial codes. Reading through supply chain paperwork, I’ve seen some confusion stem from inconsistent labeling, so double-checking the structure and identifiers avoids costly mix-ups. Whether in analytical standards or in kilogram drums, clear naming makes a difference for compliance, audit trails, and repeat purchases.
Safety & Operational Standards
The sharp odor serves as a warning, though proper training makes for safe handling. Exposure can irritate skin, eyes, or lungs; gloves, goggles, and inert-atmosphere hoods serve as shields. Storage should separate 3-MPA from oxidizing agents, and ventilation should address off-gassing. Regulatory data highlight compliance with REACH, OSHA, and IATA standards, indicating restricted quantities and mandated safety data sheets. I’ve seen that even well-ventilated labs can accumulate trace odors, so I always recommend localized exhaust. For spills, neutralization with sodium carbonate followed by dilution lessens hazards, though it pays to prepare spill kits in advance.
Application Area
Makers of polymer resins tap 3-Mercaptopropionic Acid to build cross-linked networks for coatings and adhesives, and semiconductor companies apply it to passivate gold or silver nanoparticle surfaces. In drug manufacturing, chemists attach it to molecules via the carboxyl or thiol for prodrug development or linker assembly. Biotechnologists modify proteins and enzymes, leveraging the thiol’s high affinity for soft metals. Water-treatment specialists exploit its ability to chelate heavy metals. I’ve collaborated on projects where 3-MPA improved the performance of latex paints, acting as a dispersant or adhesion promoter. The compound’s straightforward structure translates into surprising versatility, letting it slot into both bulk industrial and fine chemical niches.
Research & Development
Research continues to probe deeper, with papers exploring its use in tailor-making catalysts, building smart materials, or enhancing biosensors. Teams work on green chemistry alternatives for manufacture, swapping out hazardous reagents or streamlining downstream purification. Chemists dig into molecular reaction mechanisms, harnessing high-throughput screening to discover new reactivity or compatibility with sensitive biomolecules. In my experience, academic and industrial R&D groups keep finding fresh uses for 3-MPA—whether in nanostructure synthesis, drug delivery, or surface modification—because its simple backbone allows for endless derivatization and adaptation.
Toxicity Research
Like most thiol compounds, 3-Mercaptopropionic Acid deserves respect for toxicity risks. Acute exposure can irritate or sensitize tissue. Animal studies highlight central nervous system effects and organ sensitivity at high doses. Chronic exposure data remain limited, though workplace monitoring programs set conservative exposure limits to avoid long-term harm. Environmental impact studies reveal potential toxicity to aquatic organisms, prompting industry to adopt careful wastewater treatment and containment. I’ve seen safety teams reinforce consistent training and medical surveillance for anyone with routine exposure. Proper labeling, clear procedural guidance, and built-in mitigations lower risk significantly.
Future Prospects
3-Mercaptopropionic Acid’s future looks promising as material science and biotechnology drive demand for reactive, customizable intermediates. With advances in polymer chemistry, interest grows around developing high-value, functional coatings using accessible linkers like 3-MPA. Bioconjugation protocols push the envelope, calling for more precise thiol chemistry to build drug conjugates and biosensors. Environmental concerns create pressure for clean, scalable synthesis processes, nudging producers to refine green production routes and recovery methods. My bet is that as tighter regulatory frameworks emerge, responsibility for safe handling and thorough toxicity evaluation will only grow more crucial. Researchers remain keen to discover new derivatives and reaction modes that leverage the full potential of this compact, powerful molecule.
A Closer Look at an Unsung Chemical
3-Mercaptopropionic acid doesn’t make headlines, but this small molecule rolls up its sleeves in many labs and factories. On the surface, it just looks like another compound—clear, with a strong odor. Dig a little deeper, and you find it involved in fields from plastics to medicine. Few people outside of certain industries have heard of it, yet it touches many corners of modern manufacturing.
What Sets 3-Mercaptopropionic Acid Apart?
The most striking thing comes from its two reactive groups: a thiol (-SH) and a carboxylic acid (-COOH). These groups let 3-Mercaptopropionic acid snap into place during certain chemical reactions, making it valuable as a chemical building block.
In my time working on coatings for electronics, I saw chemists wrestle with surface adhesion problems. What surprised me was how something like 3-Mercaptopropionic acid could tweak a polymer’s surface. Adding a little of this acid can anchor coatings more firmly onto copper and gold, which matters inside circuit boards and tiny sensors. Factories in China, Germany, and the US have upgraded their product performance by blending in a touch of 3-Mercaptopropionic acid during production.
From Plastics to Pills
In the plastics industry, 3-Mercaptopropionic acid stands out as a chain transfer agent during polymerization. It helps control polymer length and branching, important details that influence how plastics bend, stretch, or resist breaking. Pretty much every industry that makes specialty plastics—think medical tubing, paint films, or flexible cables—has looked into using this compound.
The story doesn’t stop at plastics. Over the past decade, pharma researchers have dug up new uses for thiols. 3-Mercaptopropionic acid plays a part in making certain drugs smoother to produce. It pops up as a reagent to protect or “cap” sensitive groups on drug molecules, making sure those drugs hold up under tough conditions. In one project I followed, failure to protect some groups early in synthesis wasted months of work. Adding 3-Mercaptopropionic acid saved countless headaches. That kind of problem-solving shows why researchers trust it.
Environmental and Safety Notes
Every tool has risks, and thiol-based chemicals like this one are infamous for their sharp odors and tendency to irritate skin or eyes. It pays to treat this stuff with respect, wearing gloves, goggles, and working in a good hood. One batch that leaked onto a lab coat lingered for days, a reminder that careful handling goes a long way.
Waste concerns show up too. Teams have grown more aware of local rules for disposing sulfur compounds. Some labs recycle used solvents, others neutralize and dilute wastes before sending them out. The drive for greener chemistry has led to efforts to use smaller amounts, and some groups experiment with safer substitutes. Still, 3-Mercaptopropionic acid keeps earning its place thanks to its reliability.
The Road Ahead
With new electronics, wearable sensors, and sustainable plastics on the rise, demand for building blocks like 3-Mercaptopropionic acid isn’t dropping anytime soon. Chemists keep finding clever ways to fit it into reactions, and manufacturers depend on its reliability. As with most chemicals, the magic lies not in the compound itself, but in the skill and care of those who use it. Every time an engineer solves a problem with it, 3-Mercaptopropionic acid proves why it’s earned its odd place behind the scenes.
Breaking Down the Structure
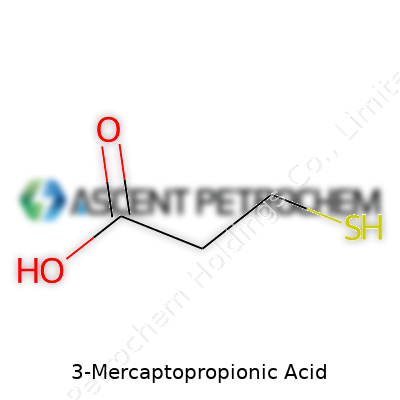
3-Mercaptopropionic acid is a molecule that keeps things simple but packs a punch in the world of organic chemistry. Its chemical formula, C3H6O2S, points straight to its makeup. Here, you have a three-carbon backbone that connects three key groups: a carboxylic acid (-COOH) at one end and a thiol (-SH) group at the other. The structure lays out as HS–CH2–CH2–COOH.
As someone who has gotten lost in the maze of organic molecules before, I can say that 3-mercaptopropionic acid stands out by keeping its architecture simple but incredibly useful. The molecule’s backbone and side groups help it bridge the world of water-loving and fat-loving environments. That split personality explains why it turns up in so many applications, from biochemical research to industry.
Where the Structure Matters Most
The story here isn’t just about lab notes or exam answers. The thiol group (-SH) sets it apart. Thiols tend to react with heavy metals and proteins, opening doors to lab experiments that search for enzyme functions, or treatments to capture metal ions and keep ecosystems safe. The other end, the carboxylic acid, helps anchor the molecule in larger systems—think of how it can bind to other chemicals, or slip onto surfaces to make new materials.
I remember a time working with protein purification, where 3-mercaptopropionic acid’s thiol end hooked onto metal beads, acting like a lock and key. This property let us separate molecules that you usually couldn’t get apart with water and salt alone. That small structural detail—the placement of the sulfur atom—often meant the difference between frustration and clear results.
Why Its Simplicity Drives Utility
One thing I noticed after years of seeing what works in real-world labs: simplicity is underrated. The three-carbon chain in 3-mercaptopropionic acid gives enough distance between the functional ends without getting tangled. As a result, reactions move forward more cleanly, whether someone is modifying biomolecules or building plastics.
The facts bear this out. Research has shown that 3-mercaptopropionic acid acts as an effective chain transfer agent in polymer chemistry, controlling the size and branching of synthetic materials. In environmental chemistry, scientists use this molecule to bind mercury and other toxic metals, signaling trouble long before people see it. It’s not the flashiest molecule, but its structure puts it to work day in and day out.
Facing Challenges and Pushing Forward
Despite this versatility, working with thiol compounds calls for care. The distinctive sulfur smell and the risk of oxidation mean proper storage and ventilation matter more than most realize. Problems have come up in facilities that underestimated the odors or let the thiols degrade. Training workers and investing in good safeguards keeps things running—cost-effective moves that prevent headaches down the line.
In biotechnology, researchers have started exploring ways to tweak the structure, adding side chains or changing the backbone to suit demands. This kind of innovation can boost selectivity for medical diagnostics, help clear up water pollution, and even improve the surface chemistry of sensors. Making small changes to the core structure opens new avenues every year, showing how this humble molecule continues to punch above its molecular weight.
Getting to Know the Risks
3-Mercaptopropionic Acid shows up in many labs and specialty industries, especially in the world of polymers and organic synthesis. Anyone working with it has likely caught a whiff of that sulfur smell—it’s no secret that this isn’t a gentle compound. Skin and eye contact can cause real irritation. The risk stretches beyond simple discomfort. This compound gets absorbed quickly if it lands on skin, causing redness, sometimes burns. Splashing into eyes stings, and careless inhalation of vapors may leave someone coughing, short of breath, or with headaches. Ingestion ranks as a medical emergency, leading to nausea or central nervous system problems.
Controlling Exposure
I’ve watched new lab staff hurry through safety checks, eager to get experiments running. Too many don’t realize that good habits today prevent trips to the emergency room later. Proper gloves matter: nitrile stands up best. Latex breaks down too fast with organosulfur. Eye protection deserves respect—goggles, not regular glasses, keep splashes out. Avoiding open skin is just common sense, so lab coats and closed-toed shoes mean less risk. Good habits prove themselves with age: even veterans keep the same routine.
Fume hoods tame most vapor risks. 3-Mercaptopropionic Acid may not fume like hydrochloric or nitric, but one careless pour can kick up enough vapor to irritate the lungs. No one wants to risk bronchial spasms or a coughing fit mid-experiment. Keeping containers tightly shut prevents accidental exposure and keeps the smell under control.
Storing Chemicals Safely
Acid storage setups feel boring, but they save headaches later. This acid reacts fiercely with oxidizers and bases. Storing strong acids and bases together on a shelf sounds efficient—until a spill mixes them. A simple plastic tub or dedicated acid cabinet makes things easier and lowers risk across the board. Keep this compound cool and away from shared refrigerators used for food or drink. Out-of-sight means forgotten, and forgotten chemicals turn into disaster after a power outage or leak.
What to Do if Something Goes Wrong
Quick responses save skin and eyes. If a splash happens, wash right away—fifteen minutes for eyes or serious skin exposure. Small exposures demand respect too. If anyone feels unwell, get them fresh air. Breathing support and medical help might be necessary if symptoms don’t stop. Swallowing needs an emergency call, not guesswork. Hazmat crews train for bigger spills, but even small leaks should be covered with inert absorbents and collected for disposal. Dumping down the drain causes problems downstream in water supplies and sewer pipes.
Building Safer Workspaces
Labels, training, and open conversations build trust in a lab. People shouldn’t have to get hurt to pay attention. Each year, hundreds of lab workers visit clinics with chemical burns or poisoning. OSHA, NIOSH, and the CDC lay out clear guidelines—good labs display them and review them as part of routine meetings. Making sure new staff understand real-world risks, not just rules on a wall, protects everyone. Chemicals like 3-Mercaptopropionic Acid don’t forgive mistakes, but strong habits and teamwork keep mishaps rare.
Spotting the Different Purity Levels
3-Mercaptopropionic acid doesn’t often catch headlines outside chemistry circles, but anyone dealing with lab work or manufacturing, from polymers to pharmaceuticals, recognizes its value right away. Purity can decide the entire outcome in a synthesis, so it makes sense that buyers want to know exactly what they are working with. Most of the bottles or drums on the market show a label reading “98% purity” or sometimes higher, right up to “99%+.” These numbers might seem trivial, but that tiny 1%—contaminants, oxidized forms, water—could throw off a reaction or result in batches failing quality control.
Why Small Purity Variations Matter in Practice
A lot of specialty chemical suppliers target that 98% range because it balances cost and quality. In my own grad lab days working on cross-linked polymers, using slightly lower grade 3-mercaptopropionic acid could mean weeks wasted on troubleshooting. The extra cost pays off compared to the work lost from impurities sabotaging a reaction. Pharmaceutical teams push even further, demanding “analytical grade” or “high purity” (99% and above), since trace impurities risk patient safety or regulatory headaches. These higher purities usually come with full certificates of analysis (COA) spelling out heavy metals, water content, and residue on ignition.
The Grades Available: Technical, Laboratory, and High Purity
Technical grade 3-mercaptopropionic acid often heads into industries like adhesives or water treatment, where tiny impurities don’t disrupt the process. This grade comes in around 97-98% purity. Laboratory grade or reagent grade commands higher prices because chemical reactions depend on predictable, clean performance. For those in R&D or quality control, this guarantees more consistent results. High purity or analytical grade sees use in pharmaceutical or high-performance polymer markets. I remember a colleague in bioconjugation chemistry whose entire week’s work would be invalidated if an HPLC run uncovered a hidden contaminant.
Quality Control: Not Just a Piece of Paper
Most reputable brands provide a COA with each batch. I always check for peroxide content and water analysis—these sneak into older or improperly stored 3-mercaptopropionic acid. Peroxides neutralize thiols, taking 3-mercaptopropionic acid out of the pool before synthesis even starts. Even a reputable supplier can slip up; once, our storage room’s humidity ruined an expensive sample in a few days. Moisture can soak through loosely capped drums or plastic bottles, dropping purity in storage. It drives home that buying high purity also means keeping it that way with careful storage—dry, cool, sealed tight.
What Buyers Should Look For
Choosing the right grade comes down to project stakes. Academia might risk technical grade for cost, but any commercial product with customers at the end needs a tighter spec. I always cross-check not just the main purity value, but also residue, heavy metals like iron and copper, and sulfur content. Suppliers who supply detailed batch reports earn my trust because trace elements crop up in unpredictable ways. No scientist wants to build a week of work around an acid that’s already halfway oxidized when it arrives.
Moving Toward Reliable Supply
Price wars rarely favor users needing highest purity. Customers focused on reproducibility and safety look for clear documentation, and often conduct their own purity checks after delivery. Chemical sourcing isn’t glamorous, but the wrong grade can derail innovation or lose trust with clients. In my experience, the best investments go toward suppliers with a reputation for consistency, documentation, and transparent answers about trace impurities.
Understanding What’s at Stake
I remember the startled look on my colleague’s face during my first year working in a chemical lab. A small bottle leaked, filling the room with a stinging smell. That bottle held 3-Mercaptopropionic Acid. Anyone who’s worked with it knows: improper handling turns routine workday into hazard control. Fast-forward a few years, I see the biggest problems come not from malice, but from carelessness and oversight. Safe handling always starts with respect for the risks—skin corrosion, strong odor, and flammability. No one wants to end up in an eyewash station.
Conditions That Actually Work
Acid like this reacts fast with bases and oxidizers. I’ve seen expensive stock ruined and safety put at risk from one missed detail. Metal containers invite corrosion. Storing it in high-density polyethylene (HDPE) or glass jars with tight seals lowers risk, and keeps out water that triggers nasty fumes. A well-ventilated, cool dry cabinet—not mixed with acids, peroxides, or oxidizers—cuts down on health risks. Everyone in the supply chain, from lab technician to truck driver, needs that reminder: separate acids and bases.
Real Risks During Shipping
Regulations around 3-Mercaptopropionic Acid transport aren't just bureaucratic hurdles—they save lives. Containers often leak because they were refilled using a funnel still wet with another chemical. That’s no rare mistake. Seasoned transport managers double-bag containers and secure them upright on pallets, with clear “corrosive” and “toxic” labels. If you drive a commercial vehicle, you’ve probably read safety data sheets that spell out wearing gloves, safety eyewear, and knowing where to find spill kits. These routines come from harsh lessons, not paranoia.
Protecting People and the Planet
Spills don’t just burn skin—they can seep into drains and end up polluting rivers. I’ve seen reports where mishandling led to months-long soil cleanup. Good companies install chemical-resistant flooring and keep absorbent materials handy. If a leak occurs, trained staff neutralize the spill and wear proper gear. These steps keep neighbors out of harm's way and avoid messy lawsuits.
Building a Safety-First Culture
Knowledge beats fear. Regular training on chemical handling, from correct PPE to emergency response, empowers everyone who handles hazardous acids. I’ve seen attitudes shift when people know what and why, not just how. Digital inventories and real-time monitoring help spot expired stock or temperature swings, long before they become problems. It doesn’t take big budgets—a disciplined checklist catches most preventable errors.
Lessons Learned in the Field
From warehouse to delivery truck, trust builds on shared responsibility. After years in the industry, I can say that culture matters as much as the chemical formula. Listening to senior staff, investing in storage containers that actually last, and reviewing near-misses teach more than a hundred memos. Respect for the risks of 3-Mercaptopropionic Acid isn’t optional—a missed detail can cost a career, or a life. Treat storage and transport as everyone’s job, not just the safety manager’s.
| Names | |
| Preferred IUPAC name | 3-sulfanylpropanoic acid |
| Other names |
Thiopropionic acid
3-MPA β-Mercaptopropionic acid 3-Sulfanylpropanoic acid |
| Pronunciation | /θriː-mərˌkæp.təˈproʊ.pi.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 107-96-0 |
| 3D model (JSmol) | `3d_006060jsmol` |
| Beilstein Reference | 1057632 |
| ChEBI | CHEBI:86415 |
| ChEMBL | CHEMBL50278 |
| ChemSpider | 311 |
| DrugBank | DB01944 |
| ECHA InfoCard | DTXSID3023762 |
| EC Number | 3.1.4.4 |
| Gmelin Reference | 8937 |
| KEGG | C00956 |
| MeSH | D008076 |
| PubChem CID | 7190 |
| RTECS number | TZ6010000 |
| UNII | 17H4N45T1U |
| UN number | UN2689 |
| Properties | |
| Chemical formula | C3H6O2S |
| Molar mass | 106.14 g/mol |
| Appearance | Clear colorless to yellow liquid |
| Odor | Unpleasant, strong, garlic-like |
| Density | 1.21 g/mL at 25 °C |
| Solubility in water | Miscible |
| log P | -2.2 |
| Vapor pressure | 0.03 mmHg (25°C) |
| Acidity (pKa) | 4.34 |
| Basicity (pKb) | 11.52 |
| Magnetic susceptibility (χ) | -46.41 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.507 |
| Viscosity | 25 mPa·s (25°C) |
| Dipole moment | 3.1092 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 192.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -482.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -724.6 kJ/mol |
| Hazards | |
| Main hazards | Corrosive, harmful if swallowed, causes severe skin burns and eye damage, toxic to aquatic life. |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes severe skin burns and eye damage. |
| Precautionary statements | P210, P233, P280, P301+P312, P305+P351+P338, P330, P501 |
| NFPA 704 (fire diamond) | 3-1-0 |
| Flash point | 144 °C |
| Autoignition temperature | 242 °C (468 °F; 515 K) |
| Lethal dose or concentration | LD50 Oral Rat 135 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 160 mg/kg |
| NIOSH | RN 107-96-0 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 3-Mercaptopropionic Acid: Not established |
| REL (Recommended) | GLC20701000 |
| IDLH (Immediate danger) | 250 ppm |
| Related compounds | |
| Related compounds |
Thioglycolic acid
Cysteine Glutathione 2-Mercaptoethanol Mercaptoacetic acid Dithiothreitol Cystamine Homocysteine |
