Dimethyl 3,3'-Thiodipropionate: Beyond the Basics
Tracing the History of Dimethyl 3,3'-Thiodipropionate
Long before technical data sheets and regulatory filings, chemical industries chased solutions for aging rubber and plastics. Dimethyl 3,3'-Thiodipropionate, born from the need to slow down the relentless attack of oxygen on polymer chains, grew popular across manufacturing hubs in the latter half of the twentieth century. Early methods for making this compound, often rough in their execution, got refined as researchers demanded higher purity and more consistent performance. In the lab and on the shop floor, tracking oxidation led to fierce competition—not just for speed but for reliable, reproducible outcomes. Proven in tire plants, wire coating lines, and packaging laboratories, this molecule carved out a meaningful place by helping products last longer and perform better under stress.
Understanding the Product
Dimethyl 3,3'-Thiodipropionate shows up as a colorless to pale yellow liquid when pure, sometimes forming a fine crystalline solid at lower temperatures. Its faint, sweetish odor sneaks through when uncapped, hinting at its chemical roots. In most settings, bottles carry clear warning labels; safety comes as much from training as from alarms. Most batches measure above 98% by assay, with moisture kept low to prevent hydrolysis that would spoil its functionality. Industrial buyers care about the subtle difference a fraction of a percent can make for batch consistency and cost control, so specifications get tight, batch records thicker, and trust more valuable.
Physical and Chemical Properties: What Matters on the Ground
The backbone of Dimethyl 3,3'-Thiodipropionate centers on the thioether bond, with its sulfur atom bridging two propionate chains capped by methyl esters. The molecular weight holds steady at about 210 grams per mole. Boiling points sit near 140–150°C at reduced pressure; above that, decomposition creeps in. Its solubility in many organic solvents—acetone, ethanol, toluene—means it can go right into common formulations without much fuss, yet it stays almost completely out of water. Reactivity often stays manageable unless exposed to strong bases or acids, and keeping it cool, dry, and sealed makes a big difference for shelf life.
Technical Specifications and Labeling: Speaking the Same Language
Anyone moving this chemical from factory to customer counts on numbers and safety codes. Certified lots carry assay, melting and boiling points, specific gravity, refractive index, and maximum allowable acid or water content. Shipping containers feature the CAS number 123-67-1, GHS pictograms for irritants or environmental hazards, and batch-specific traceability. Proper labeling means lab technicians can store, use, and dispose of products safely, and that regulators can trace material in the unlikely event of an emergency or contamination.
Manufacturing and Preparation: Keeping it Pure
The typical way to make Dimethyl 3,3'-Thiodipropionate runs through the esterification of 3,3'-thiodipropionic acid using methanol in the presence of an acid catalyst, such as concentrated sulfuric acid. The process requires careful temperature control: too hot, and side reactions take over; too cold, and reaction times drag out. Water produced during this reaction can stall conversion if not removed, so chemical engineers use azeotropic distillation or drying agents to draw it away. At the end, neutralization and multiple washings weed out any traces of acid before distillation gives the final pure product. Every tweak in process changes the impurity profile, and mistakes can damage downstream polymers or activate regulatory headaches.
How Chemists Tweak and React: Modification and Reactions
Chemists see Dimethyl 3,3'-Thiodipropionate as more than just an antioxidant. Its ester groups can hydrolyze under strong acid or base, regenerating the parent acid; under more extreme conditions, the thioether can oxidize to sulfoxide or sulfone, changing color and reactivity. Some have explored attaching other groups onto its backbone hoping for new performance in specialty markets. Still, in most production lines, workers look for stability rather than reactivity—this molecule’s role is to quietly catch radicals and stop chain reactions that would otherwise age rubber, polyethylene, or coatings before their time.
Other Names and Trade Labels
Dimethyl 3,3'-Thiodipropionate goes by several names depending on the supplier or regulatory context. Thiodipropionic acid dimethyl ester shows up in older literature, as does DMTDP or DMTP. A few global suppliers offer it under proprietary trade names, but the underlying molecule stays the same. Industry databases—including the European Chemicals Agency, U.S. EPA TSCA inventory, and Japanese ENCS registries—cross-link synonyms, helping buyers and safety officers match materials across borders.
Approaching Safety and Operational Demands
Anyone using Dimethyl 3,3'-Thiodipropionate knows to respect its label but not fear it unnecessarily. Splash goggles, nitrile gloves, and proper ventilation limit exposure during sampling and mixing. Inhalation rarely causes serious issues at low concentrations, but accidents with spills, vapor, or splashes push users to recognize eye and skin irritation risks. In case of fire, local fire codes recommend dry chemical or foam—not water. Safe handling depends as much on culture and training as on written rules; regular drills and up-to-date safety data sheets keep chronic exposure and preventable accidents away from daily life in the lab and on the plant floor.
Main Uses: Where It Fits in the Real World
You see Dimethyl 3,3'-Thiodipropionate in much of what people touch daily, though rarely by name. Tire makers lean on its antioxidant properties to slow the wearing out of rubber; cable insulation lines add it to plastics for heat and light resistance, keeping wires safe and flexible after years in the wall or underground. Some food packaging films use it in minute amounts to keep packages clear and strong without adding taste or smell. Paint labs and hot-melt adhesive researchers count on it to stabilize formulas exposed to sun or heat, delivering longer life and better performance out of every batch. The value in these cases comes not from dramatic effect but from quiet protection; lives, property, and brand reputation all hinge on stability at the molecular level.
Research, Rethinking, and What’s Next
Scientists in both academia and industry keep poking at how Dimethyl 3,3'-Thiodipropionate works under different temperatures, pressures, and light exposures. Polymer researchers want to move to greener chemicals or lower-dose systems, but few additives show the same broad compatibility with tough polymers and pigments as this one does. A trend shows up in patents and research papers: blending Dimethyl 3,3'-Thiodipropionate with other stabilizers for a cumulative effect, or using it to trap specific radicals in next-generation bio-based materials. Regulatory changes sometimes push the lab’s focus, with lower permissible exposure limits or tighter limits for food contact materials in Europe or North America.
Understanding Toxicity and Health Research
Toxicologists have been tracking Dimethyl 3,3'-Thiodipropionate across decades of rodent and workplace studies. Acute toxicity runs low, meaning one-off accidental exposures cause limited immediate harm compared to more reactive chemical cousins. Chronic exposure at high vapor or dust concentrations, though, prompts irritation in the lungs and eyes, demanding respect for ventilation and PPE. Environmental studies so far suggest moderate persistence in the environment, with slow break-down under aerobic conditions. Regulators still watch for signs of bioaccumulation or subtle long-term health effects, prompting ongoing review, especially for food packaging and children’s toys.
Looking Ahead: Opportunities and Challenges
Traditional uses of Dimethyl 3,3'-Thiodipropionate still dominate sales reports, but changing consumer priorities and regulatory trends could shape its future. Green chemistry labs look for ways to source starting materials from plants or waste streams, reducing dependency on oil and improving lifecycle numbers. New application areas—think energy storage films, advanced textiles, or recyclable packaging—create demand for old additives used in new ways. At the same time, competition from novel antioxidants and a relentless focus on cost, safety, and sustainability will keep producers, buyers, and regulators alert. Every batch in the future stands not just as a product but as a reflection of chain-of-custody, safety, and innovation, connected directly to the lives and industries counting on polymers that last longer and perform better under pressure.
What Makes This Chemical Count
Dimethyl 3,3'-Thiodipropionate pops up mostly in the plastics industry. Some names do not roll off the tongue, but their purpose shows up all over our daily routines. You get a plastic water bottle or a stretchable food wrap, and what you don’t see are all the additives packed inside. These extras help these items survive heat, last longer on a store shelf, and hold up against rough use. This particular chemical acts as an antioxidant in plastic manufacturing. It helps plastics fight off the damage from heat and air during their creation and while sitting on shelves or in someone’s home.
Take PVC, for example. This plastic gets used for everything from pipes to kids’ toys. Without some sort of protection, those products grow brittle, turn yellow, or fall apart over time. Dimethyl 3,3'-Thiodipropionate steps in to help slow down that decay. The science is pretty clear: antioxidants mop up the invisible attacks from oxygen, stopping plastic from breaking down so quickly.
Everyday Connections: Why This Matters to More Than Just Chemists
Today’s families depend on plastic for food safety, for plumbing, for packaging that keeps strawberries fresh on the ride home from the store. As someone who tries to keep leftovers fresh and kids’ toys in the toy box instead of the trash, the idea that small changes in a recipe can keep products safer for children hits close to home. Using antioxidants like Dimethyl 3,3'-Thiodipropionate means companies offer tougher, longer-lasting goods.
That focus on durability does more than save money for families. It touches the planet, too. If a plastic pipe lasts longer, no one needs to rip it out and throw it away so soon, meaning fewer replacement cycles and less landfill waste. The less we replace and toss out, the better for all of us. The same logic works for household containers, automotive parts, and electronics.
Keeping an Eye Out for Safety and Solutions
Every time an additive like this enters a supply chain, safety must stay in the picture. Regulatory agencies, including the US Environmental Protection Agency and the European Food Safety Authority, study these chemicals. They keep close tabs on how much ends up in the environment and whether it could harm people or wildlife. Published studies on Dimethyl 3,3'-Thiodipropionate fall in line with current limits, though the industry keeps changing and more research always makes sense.
Looking down the road, better chemicals could come along, or ways to recycle plastics might make these helpers less needed. Plenty of scientists look for natural replacements and better recycling systems, but right now, Dimethyl 3,3'-Thiodipropionate fills a real need.
Ask parents, plumbers, or manufacturers about quality and shelf-life, and you’ll hear stories about broken toys, burst pipes, or spoiled food nobody wanted. Those headaches shrink with the right tools in the toolkit. As more people ask questions about what goes into the things they buy, transparency, more research, and smarter innovation will keep making the world a bit safer and a lot less wasteful.
Some Chemicals Need Respect
Dimethyl 3,3’-Thiodipropionate isn’t on most people’s radar, but for folks in the plastics or coatings world, it turns up as a stabilizer and makes life easier. The flip side—being around it—demands a bit of caution. I’ve seen people let their guard down with unfamiliar compounds, but chemicals always “pay you back” for that mistake. Personal experience in a lab setting reinforced the basic truth: a relaxed approach leads to unintended messes, sometimes hazardous ones.
Personal Protection Comes First
Anyone who’s handled organosulfur compounds knows their scent lingers, even after the job seems done. Breathing protection matters, specifically when powder or vapor may escape. Simple, well-fitted respirator masks filter out nuisance fumes well enough. Nitrile gloves keep bare skin untouched, and a pair of tight-fitting safety goggles guards against splashes. Cotton sleeves keep stray dust from reaching your arms; I’ve watched enough colleagues develop rashes to call it essential. Never substitute basic PPE with “just being careful.”
Clear Spaces and Fresh Air
Work only in places with a fume hood or steady airflow. Even if technical data notes low toxicity, sulfur compounds hang in the air and can start headaches or cause eye irritation. Windows open wide, fume hoods running on high, keeps everybody in good shape. Stuffing a project into a tight space just makes accidents harder to control.
Good Habits Beat Disasters
Every workplace has its “just wash it down the sink” folks. Washing chemicals like Dimethyl 3,3’-Thiodipropionate into the drain creates long-term problems. Even trace residues in groundwater or pipes risk harming animals and set off regulatory headaches. Safe habits—closed containers, labeled bottles, spill kits—stop most trouble before it starts. Quick cleanup matters: one clumsy elbow or upturned flask can send chemicals everywhere. Paper towels, absorbents, and neutralizing agents should stay within arm’s reach.
Storage Isn’t Boring—It’s Safety
I used to think “cool, dry, dark place” could mean anywhere except near a radiator, but stable storage really means much more. Store this compound in sealed, shatterproof bottles. Keep it away from acids and oxidizers. If you store it next to anything flammable, you’re asking for trouble: reactions, releases, and sometimes fire follow poor organization. Labeling bottles with hazard symbols and contact info can save hours in an emergency.
Training Means More Than a Checklist
People sometimes treat chemical training as a box to check, but real awareness only comes from experience and curiosity. Taking time to learn the safety data sheets, asking “what does this do if spilled?”, and walking through emergency gear locations turns nervous newcomers into skilled handlers. Nobody should face a spill alone or in the dark. Responsible teams talk through risky scenarios and review actual near-misses, not just the paperwork.
Small Details Keep People Safe
Filling spill logs, restocking gloves, and checking fire extinguishers never feel thrilling, but those routines hold the safety net in place. Chemical exposure leaves a long memory. A friend of mine skipped goggles just once, and a week of red, painful eyes changed his entire attitude about lab work. The best safety culture comes from people who remember close calls and help others avoid them.
Everyday Vigilance Protects Everyone
Handling chemicals never turns into a routine job. Every bottle holds a potential lesson. Staying prepared—both with PPE and with practical knowledge—gives confidence and keeps unwelcome surprises away. Keeping everyone safe relies on the same habits you’d want a colleague to hold if you couldn’t be there.
A Closer Look at the Molecule
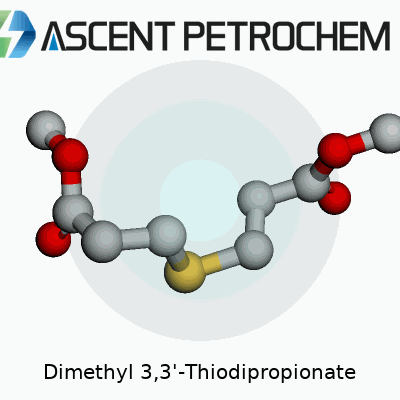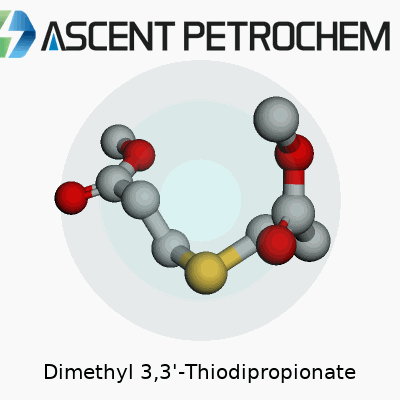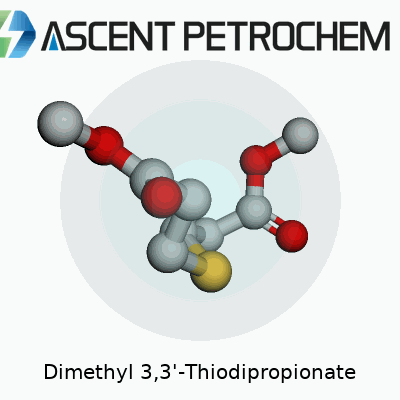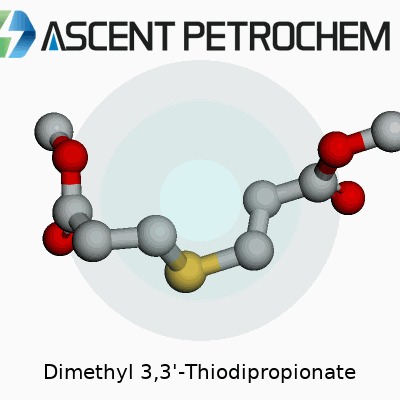
Dimethyl 3,3'-Thiodipropionate sounds like a mouthful, but at its core, this compound is about as straightforward as a molecule can get once you break down its name. Take a sulfur atom and tether it between two three-carbon chains. At the ends of those chains, attach methyl ester groups. Chemists know this structure as C8H14O4S. Line it out, and you’ll spot SCH2CH2COOCH3 on both sides. That “thio” link—essentially a sulfur bridge—gives the molecule both strength and a unique set of chemical properties.
Being around labs and factories, I’ve seen this molecule show up as a white powder or clear liquid. It doesn’t have a strong odor, unlike other sulfur compounds. The methyl ester “caps” at each side improve its resistance to hydrolysis, so it’s less likely to break down when exposed to water compared to plain diesters. This stability matters a lot in tough environments.
Uses Built on Structure
People trust Dimethyl 3,3'-Thiodipropionate in polymers for a reason. The sulfur sitting between the two propionate groups acts like a shield. It scavenges free radicals, neutralizing the molecules that trigger breakdown in plastics, rubbers, and other materials exposed to heat or oxygen. So its shape and chemical bonds aren’t just for show—they decide how well it works as an antioxidant. You see bags, cords, and even plastic covers last longer because of this small but crucial molecule.
Efforts to keep food from spoiling also rely on molecules with similar sulfur bridges. In food packaging, for instance, plastic layers stop oxygen from sneaking in and ruining the product. Additives like Dimethyl 3,3'-Thiodipropionate slow this decay. The chemistry behind this is pretty simple: the thioether group steps in to mop up the reactive oxygen species, letting the packaging do its job much longer.
Safety and Environmental Questions
Chemical stability, while good for the end product, raises questions about long-term safety. A molecule resistant to breakdown lasts a long time outside, so if plastic bits or fragments containing this compound end up in soil or water, they might stick around. Studies tracking how it moves and breaks down in the environment draw from its structure: that thio- bridge holds up against many forms of natural attack, which can be a double-edged sword.
In manufacturing plants, strict rules surround its handling. Those ester groups and the sulfur bridge don’t vaporize into the air easily, so most risks show up in accidents and spills rather than in steady exposure. Safety data sheets make it clear: gloves and goggles matter. I’ve worked with operators who experienced skin irritation after direct contact, which makes personal protective equipment a daily habit around this compound.
What’s Next for Dimethyl 3,3'-Thiodipropionate?
It’s easy to gloss over the role a single molecule plays in keeping materials from crumbling or products fresher for longer. Dimethyl 3,3'-Thiodipropionate stands out not by chance, but because its structure—those ester groups, the thioether bridge—does serious work. As demand for more durable plastics and better food safety rises, research pushes towards additives that do similar jobs but with easier breakdown after use. Bio-based analogues and greener antioxidants may someday replace this compound, but for now, the chemistry gets the job done in a way that’s hard to beat.
Understanding the Chemical’s Nature
Dimethyl 3,3'-Thiodipropionate shows up in the plastics and polymer world as an antioxidant, helping materials resist heat and aging. From its distinct sulfur odor to its low melting point, this chemical brings certain risks that deserve respect. I’ve worked in labs where one careless mistake around such compounds set off headaches nobody wanted—from regulatory headaches to actual ones among staff.
Risks and Realities of Handling
Any warehouse manager or lab technician who’s dealt with sulfur-containing esters knows what I’m talking about: leaks sink into walls, and a warm room spells disaster. Research from occupational safety groups and chemical safety boards keeps warning us—improper storage easily leads to economic and health consequences. These risks go beyond losing a batch; they touch worker safety and public trust. According to the U.S. Occupational Safety and Health Administration, accidental exposure and improper ventilation often drive reported incidents.
Good Storage Practices: My Experience on the Ground
Let’s cut to real lessons learned. Keep Dimethyl 3,3'-Thiodipropionate in airtight, sealed containers to trap the pungent vapor. Glass works, though chemical-grade HDPE stands up fine too. I have seen corrosion around cheap metal lids, so I don't recommend them no matter how convenient it seems. Store it somewhere cool and dry, away from bright sunlight or hot rooms. Every supply room I’ve set up focused on keeping chemicals behind fireproof cabinets—safety built-in from the start.
Ventilation counts. Strong, steady airflow flushes out lingering fumes. I remember one plant where the storage area had no vents—within weeks, minor fumes crept into adjoining offices. Installing fans made an immediate difference, both for daily air quality and in keeping workers’ trust intact.
Labeling and Segregation for Safety
Labels should be large, legible, and waterproof. Our teams use a two-label system: manufacturer’s info plus a big pictogram showing main hazards. Storing this compound away from acids, oxidizers, and heat sources shrinks the risk of runaway reactions. Mixing storage with bleach or peroxides leads to unpredictable outcomes, so we always kept separate cabinets for incompatible materials.
Emergency Readiness: Learning from Mistakes
Emergencies don’t warn you ahead of time. A spill response kit belongs within reach, filled with gloves, absorbent pads, and neutralizing materials. In a dusty lab corner years ago, a cracked container leaked—quick action prevented a much larger cleanup. I’ve noticed that clear, simple training makes all the difference. Annual drills drive home proper cleanup and evacuation routes. This proactive approach lines up with guidance from the EPA, which highlights clarity and consistency in response plans as keys to preventing environmental releases.
Adapting for Long-Term Safety
Long shelf lives tempt some facilities to stockpile. Regular checks spot cracked seals, discolored liquid, and condensation inside containers. Prompt action keeps the stash in good shape. In every job, we’ve had a checklist—simple, low-tech, but kept up-to-date by people who know the real value of vigilance. Partnering with safety officers ensures that protocols stay current, especially as industry guidelines adapt. Everyone plays a part: from procurement, to storage, to disposal.
Smart storage of Dimethyl 3,3'-Thiodipropionate isn’t just regulatory box-checking. It protects real people, property, and our community. With common sense measures, steady habits, and solid teamwork, facilities keep the risks in check and focus on their day-to-day work without worry.
What Is Dimethyl 3,3'-Thiodipropionate?
Dimethyl 3,3'-thiodipropionate usually turns up in factories, especially where plastic products get made. Its main job is to keep plastics from breaking down, especially under heat, oxygen, or sunlight. This chemical does not go by a catchy nickname, but it quietly supports the modern world where packaging, automotive parts, and electronics need to last longer.
Health Perspective
No chemical gets a free pass. Anyone who has spent time in an industrial lab knows how carefully one needs to handle anything that doesn’t come straight from the kitchen shelf. For dimethyl 3,3'-thiodipropionate, most research points to low acute toxicity. Animal studies show high doses cause irritation in the eyes and on the skin. Standard lab gloves, goggles, and fume hoods are enough to keep these effects at bay. Still, no one should brush off the fact that chronic exposure stories remain thin on the ground. Chemicals with sulfur bonds can sometimes break down in ways that produce new hazards if workers breathe them in or spill them on their clothes.
User safety always deserves more than just the manufacturer’s assurance. Researchers suggest staying ahead of any chemical risk by reviewing workplace air testing data and updating training whenever a new study comes out. Without solid, long-term studies on how dimethyl 3,3'-thiodipropionate behaves after a decade of use, it makes sense to approach handling it with every layer of protection the workplace can provide.
Environmental Impact
The leap from the factory floor to the surrounding air or water raises another batch of questions. Reports to the European Chemicals Agency show low bioaccumulation potential and no evidence for widespread persistence in soil or rivers. So far, natural bacteria can break this molecule apart faster than some tougher plastic ingredients. That doesn’t mean tossing excess powder down the drain is ever smart.
I remember a local case near a plant in central Ohio where a chemical with a similar sulfur structure led to a sudden drop in aquatic worms in drainage ditches. Cleanup lasted for months. No one linked any major event to dimethyl 3,3’-thiodipropionate specifically, but sulfur-based breakdown products can tip an ecosystem even when labeled “low hazard.” If release happens, risk managers don’t focus only on what the original product does. They also check for what new compounds sneak out in runoff, especially in habitats that bounce back more slowly.
Managing the Risks
For businesses and regulators, the real priority comes down to prevention and transparency. Chemical manufacturers who stick with closed-loop handling and enforce spill reporting keep the air and water safer for neighbors and for their own employees. Public databases, like the EPA’s Toxics Release Inventory, encourage companies to report how much dimethyl 3,3'-thiodipropionate they use and where it ends up. Communities weigh their exposure risks by digging into that data. If residents suspect higher-than-average chemical levels, third-party labs can run air, soil, and water checks to firm up the numbers.
Workers and communities both benefit from more science. Funding independent, ongoing studies gives everyone a clearer picture. Local advocacy and health groups do good work when they push for clearer labels, stricter spill protocols, and transparency on long-term studies. Real safety grows from honest, regular reporting, not from the claim that “no proven hazards exist.”
Looking Forward
No single plastic additive will disappear from the market overnight. Until more evidence piles up, caution and open reporting stand as the best answers for keeping risks contained. It pays to listen to researchers, workers, and residents on the front lines, because they spot problems quickest and press hardest for accountability.
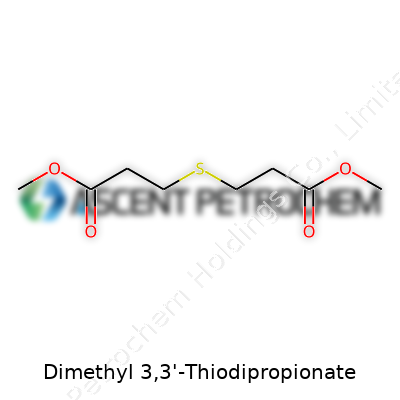
| Names | |
| Preferred IUPAC name | Dimethyl 3,3'-sulfanediyldipropanoate |
| Other names |
Dimethyl thiodipropionate
Dimethyl 3,3-thiodipropionate Thiodipropionic acid dimethyl ester DMTP Methyl 3-(methoxycarbonylthio)propanoate |
| Pronunciation | /ˈdaɪˌmɛθəl θaɪˌoʊdaɪˈprɑːpɪəneɪt/ |
| Identifiers | |
| CAS Number | 123-28-4 |
| Beilstein Reference | 322086 |
| ChEBI | CHEBI:84315 |
| ChEMBL | CHEMBL4354891 |
| ChemSpider | 21240 |
| DrugBank | DB16643 |
| ECHA InfoCard | ECHA InfoCard: 100.015.299 |
| EC Number | 123-28-4 |
| Gmelin Reference | 83478 |
| KEGG | C18606 |
| MeSH | D021239 |
| PubChem CID | 8444 |
| RTECS number | WY5250000 |
| UNII | YZG6H9E89O |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | `DTXSID4020428` |
| Properties | |
| Chemical formula | C8H14O4S |
| Molar mass | 210.29 g/mol |
| Appearance | White crystalline powder |
| Odor | Mild characteristic |
| Density | 1.16 g/cm3 |
| Solubility in water | Insoluble |
| log P | 2.2 |
| Vapor pressure | 1.52E-4 mmHg at 25°C |
| Acidity (pKa) | 8.75 |
| Magnetic susceptibility (χ) | -62.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.485 |
| Viscosity | 28 mPa·s (25 °C) |
| Dipole moment | 3.25 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 527.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -726.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3477 kJ/mol |
| Pharmacology | |
| ATC code | '' |
| Hazards | |
| Main hazards | Irritating to eyes, respiratory system, and skin |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P261, P264, P270, P272, P273, P280, P302+P352, P333+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 134 °C (273 °F; 407 K) |
| Autoignition temperature | 320 °C |
| Lethal dose or concentration | LD50 oral rat 8,200 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral > 5,000 mg/kg |
| NIOSH | RR3150000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
Dimethyl adipate
Dimethyl succinate Dimethyl suberate Dimethyl glutarate Bis(2-hydroxyethyl) terephthalate |
