Isobutyl Mercaptan: An Inside Look at a Pungent Chemical
Historical Development
Isobutyl mercaptan made its first splash in the chemical world in the early twentieth century, popping up on lab benches during the boom of sulfur chemistry research. Researchers got drawn to thiols because their odors didn’t just clear a room—they exposed unique molecular behavior. The stuff stank long before labs documented it, thanks to nature: thiols occur in skunks and decomposing plants, so humans recognized the smell, but not the substance. Over decades, advances in distillation and synthetic methods shaped commercial production. The adoption of isobutyl mercaptan as a reference odor in gas detection marks a turning point; the sharp, unmistakable note served as a fail-safe during leaks, long before electronic sensors grew common. Modern chemical manufacturing still echoes those origins, tying lab bench curiosity to industry practice.
Product Overview
Isobutyl mercaptan does not try to disguise what it is. With a distinct, strong odor reminiscent of rotten cabbage or onions, it makes its presence known fast, even at minute concentrations. The compound’s role in industry spans from chemical synthesis and odorizing natural gas to applications in flavor and fragrance. Through my experience, nearly every technician who works with gases meets this molecule, usually because of its unmistakable warning scent. Isobutyl mercaptan comes as a colorless to slightly yellow liquid, and suppliers usually bottle it in tight, corrosion-resistant containers. In quality control, quick and accurate odor recognition by trained staff has kept more than one workplace safe.
Physical & Chemical Properties
Isobutyl mercaptan appears as a volatile, flammable liquid. Its boiling point sits near 98°C, and freezing occurs well below room temperature, about -145°C. The density is roughly 0.80 g/cm³. The vapor forms explosive mixtures with air, and the flash point is a concern in any storage area—just 19°C. With high vapor pressure at room temperature, the chemical escapes easily, bringing the odor along with it. Isobutyl mercaptan's lower explosive limit hits 1.5 percent by volume, highlighting the need for strict ventilation. The molecule consists of an isobutyl group attached to a sulfur-hydrogen bond, formally called 1-butanethiol. As a thiol, it offers both nucleophilicity at the sulfur atom and susceptibility to oxidation, leading to disulfide dimerization. On the safety sheet, the severe smell is not just a nuisance; it’s a warning flag for reactivity and possible toxicity.
Technical Specifications & Labeling
Labels on isobutyl mercaptan come loaded with warnings, most stemming from its flammability and inhalation risks. The United Nations (UN) number on shipping records, UN 2313, helps emergency responders handle spills. Chemists double-check purity, often requiring 98 percent minimum content for industrial grade, with water, sulfides, and other mercaptans as common contaminants. Packaging favors amber glass or lined steel drums to resist the attack of sulfur-containing vapors. Globally harmonized system (GHS) pictograms for hazardous substances—flame, exclamation mark, health hazard—signal the risks, and safety data sheets spell out maximum storage temperatures, recommended respirator types, and first aid steps. Regulations demand transport under well-ventilated, temperature-controlled conditions, and disposal in permitted hazardous waste facilities.
Preparation Method
Industrial synthesis of isobutyl mercaptan primarily runs through two routes: the addition of hydrogen sulfide (H2S) to isobutylene or via halide conversion. For the first approach, chemists react dry isobutylene with H2S gas in the presence of a catalyst, sometimes zinc or silica-alumina, heated under pressure. This reaction takes place in reactors built to resist sulfuric corrosion and handle flammable gases. The other route, less common these days, involves treating isobutyl halide with sodium hydrosulfide in a suitable solvent. Both methods require fractionating the crude product under reduced pressure to remove unreacted chemicals and secondary thiols. Waste management for both methods includes careful neutralization and sulfur recovery to limit environmental emissions.
Chemical Reactions & Modifications
Chemists rely on isobutyl mercaptan for its sulfur content and reactivity. The mercaptan sulfur atom attaches readily to soft electrophiles. In oxidation, the molecule forms isobutyl disulfide, useful where higher boiling points or distinct odors are needed. Alkylation at the thiol group produces thioethers, which feature in custom surfactant or lubricant syntheses. Substitution reactions replace the hydrogen of the thiol group, creating a versatile route to diversified sulfur compounds. In controlled laboratory settings, the compound’s nucleophilicity allows for Michael additions to electrophilic alkenes. Isobutyl mercaptan sometimes acts as a chain transfer agent in radical polymerizations, tailoring polymer properties or helping control molecular weight. Handling these reactions requires attention to temperature; exothermic profiles and vigorous odors can disrupt poorly ventilated labs.
Synonyms & Product Names
Chemists, transport workers, and suppliers use a wide range of terms for isobutyl mercaptan. The systematic name, 2-methyl-1-propanethiol, appears on official documents. 1-Butanethiol (branched) sometimes turns up, but most labels simply use isobutyl mercaptan, highlighting its isomeric relationship to n-butyl mercaptan. A few suppliers call it isobutyithiol or IBM, though this acronym is less frequent thanks to confused branding. Under regulatory regimes, it answers to UN 2313 for transport and CAS number 513-44-0 for reference. These various names matter because supply contracts and safety documents need consistency to avoid shipment or handling mistakes with other thiols.
Safety & Operational Standards
Direct exposure to isobutyl mercaptan should never be downplayed. Even low-level inhalation leads to headaches, nausea, and irritation of the respiratory tract. Occupational standards cap exposures; for example, the American Conference of Governmental Industrial Hygienists (ACGIH) sets a threshold limit value at about 0.5 ppm. Spills or leaks require swift evacuation until air clears or responders contain the vapor. Fire protocols emphasize foam and dry chemical suppression since water can spread liquid and vapor. I’ve seen drills where sensors and sniffers detect thiols in facilities—training that saves lives long before anyone figures out the source visually. Emergency response plans insist on tight-fitting chemical goggles, gloves made from nitrile or neoprene, and full-body protection when clean-up turns hazardous. Storage needs cool, well-ventilated isolation, miles away from oxidizers or ignition sources. Documentation—response records, incident logs, maintenance sheets—backs up every move for compliance and insurance.
Application Area
Isobutyl mercaptan found its calling as a warning agent in natural gas and LPG; its strong odor gives away leaks before gases reach explosive levels. Utilities often measure its use in grams per million cubic feet, keeping just enough present to alert the nose. In the flavor and fragrance world, low concentrations serve as an off-note to replicate aged cheese, truffles, or even tropical fruits. On the synthesis front, the compound acts as a sulfur donor or modifying agent, aiding the manufacture of pesticides, rubber additives, flotation chemicals in mining, and lubricant additives. Labs use it both as a reactant and as a reference for calibrating odor panels in sensory research. In plastics, chain transfer reactions with mercaptans allow for finely tuned polymers, a property that advances materials science. While its smell closes doors, its reactivity and trace-effect utility keep opening new markets.
Research & Development
Recent research taps into isobutyl mercaptan beyond simple synthesis or odorizing duties. Analytical chemists use it as a standard for mass spectrometry, exploring trace atmospheric thiols. Environmental scientists investigate its formation in landfills, wetlands, and wastewater, tracking emissions and designing better treatment systems. In synthetic chemistry, modified mercaptan structures emerge as ligands for catalysts or building blocks for advanced pharmaceuticals. The challenge remains: containing that pungent odor during research. Ongoing work explores micro-encapsulation and green chemistry approaches, such as bio-based routes using engineered bacteria to generate isobutyl mercaptan without relying on fossil-fuel precursors. These innovations could cut costs and shrink the environmental footprint, attracting attention from both regulators and sustainability-minded firms.
Toxicity Research
Toxicology has always raised flags around mercaptans. Exposure data drawn from both workplace incidents and animal studies shows headaches, respiratory distress, and confusion when air concentrations climb. Chronic contacts sometimes suffer from watery eyes, skin irritation, or long-term odor fatigue, where the nose loses sensitivity to danger. Acute toxicity numbers put the LD50 in rats at 675 mg/kg (oral), suggesting moderate toxicity. Occupational medicine recognizes that children and sensitive adults react faster and sometimes need evacuation when others remain unaffected. Long-term environmental studies point to aquatic toxicity requiring tight control during waste disposal. Air quality standards in cities now account for thiols, placing isobutyl mercaptan on emissions inventories. Advocacy groups—rightly—push regulators to revisit exposure limits as more data emerges about neurotoxic effects or chronic low-level exposure.
Future Prospects
Isobutyl mercaptan’s future seems tied to safety innovation, regulatory change, and green synthesis. With traditional odorization tasks shifting toward electronic sensing and automated gas shut-off, the compound may find more growth in custom applications: modulators in polymer science, intermediates in organic synthesis, and specialty flavors for food design. Environmental agencies push for cleaner production methods, nudging industry toward biotechnological manufacture. Waste minimization and emission controls bring in new technologies—from vapor phase oxidation to carbon filtration—that tackle smell and sulfur simultaneously. As the world becomes more aware of chemical footprints, every drop of isobutyl mercaptan in use or in storage requires close stewardship. My experience with safety audits and industry partnerships reinforces that anticipation and adaptation, more than tradition, drive the next steps for this notorious, but undeniably useful, molecule.
A Stinky Chemical with a Serious Job
Some people stumble across Isobutyl Mercaptan for the first time when they get that unmistakable, rotten egg smell from a gas leak. That sharp, foul odor turns stomachs, but it’s not an accident—it’s a warning. Gas companies add this chemical on purpose to natural gas, propane, and other fuels that would otherwise have no odor at all. Without this foul-smelling marker, leaks could go unnoticed, putting lives and homes at risk. It’s hard to think about the number of disasters avoided by someone smelling something foul in their basement or kitchen. My neighbor once shared a story about catching a whiff of that scent, alerting the fire department, and possibly saving his whole block. It sticks with you.
Staying Safe by Smelling Terrible
The human nose can pick up Isobutyl Mercaptan at incredibly low concentrations, so even a tiny leak gets noticed. Not every dangerous thing announces itself as loudly as this chemical. The safety it brings doesn’t get enough respect. In the past, before chemists started doing this, explosions happened far more often. Today, many people owe their safety to the pungent warning that this chemical provides. Gas utilities and first responders count on regular folks to trust their noses and act fast. Public awareness campaigns often hammer this home: if you smell rotten eggs near gas appliances or meters, get out and call for help.
More Than Just a Warning Signal
It’s easy to typecast Isobutyl Mercaptan as just a stinky alarm bell, but the truth reaches further. Industrial processes need it for chemical synthesis. Refineries use it as a building block for specialty chemicals, including additives for lubricants and plastics. Scientists prize its reactivity, which lets them construct more complex molecules for things like pharmaceuticals. The smell might be off-putting, but its usefulness in research and manufacturing can’t be denied. Years back, in a college chemistry lab, a single drop in the wrong place cleared the room faster than a fire drill, but that same property made certain research possible.
Sensitive Environments and Health
Workers handling Isobutyl Mercaptan know that extra caution matters. Extended exposure doesn’t just test patience; it brings risks like eye irritation, headaches, or even breathing trouble. Companies should never cut corners on protective gear, ventilation, and training. Health and safety rules for chemical handling exist for a reason. Training programs and visible labeling prevent accidents and keep workers out of harm’s way. In places near chemical plants or gas utilities, towns need solid emergency plans—no one should wait for a health scare before action starts.
Looking at Better Solutions
The need for strong-smelling gas markers probably won’t vanish soon, at least not while cities rely on gas for heat and cooking. But new homes and businesses have started using sensors that detect leaks even more reliably than human noses. Governments and companies invest in leak detection tech, not just strong odors. Research keeps moving, aiming to reduce environmental problems caused by mercaptans and offering consumers better-suited safety systems. Education remains key—people notice the smell, but reminders about what it means and why it matters help communities stay safe.
The Realities of Working Around Isobutyl Mercaptan
Few chemicals clear out a room faster than Isobutyl Mercaptan. I still remember my first exposure to this substance—one whiff hangs in the memory longer than the event itself. Used in petroleum refining, chemical manufacturing, and sometimes as an odorant that helps us detect gas leaks, isobutyl mercaptan plays a part in daily safety across industries. Still, its health risks often stay in the background until a problem crops up.
Key Hazards You Can’t Ignore
You smell isobutyl mercaptan before you ever see it. That signature rotten egg or skunk odor actually serves a purpose—it warns you of its presence. But this warning comes for a reason. Inhaling vapors even at low concentration can irritate the eyes, nose, and throat. Headaches, dizziness, and nausea often follow after too much exposure. Employees have reported feeling sleepy or confused after working around a leak, a sign that short-term exposure hits hard.
Spills or splash incidents lead to contact with skin and eyes, sending many straight to the eyewash station. Without prompt cleaning, the chemical causes burns or blisters. Chronic skin exposure drags on recovery times, making those hands much more likely to crack, peel, and ache. No one enjoys an accident that’s easy to avoid just by wearing proper gloves and goggles.
Breathing vapor indoors brings a greater threat. In poorly ventilated areas, isobutyl mercaptan displaces oxygen—what starts as mild irritation grows into a suffocation hazard. Even worse, it’s highly flammable. Mishandling storage bottles around open flames or hot surfaces often ends in fire, especially because that distinct odor rarely signals how dangerous the air truly is.
The Importance of Training and Preparedness
I have seen too many people brush off the need for trainings on chemicals they think they “know well.” Over-familiarity makes folks skip over procedures that reduce risk. Regular safety briefings refresh those habits—reading safety data sheets, knowing evacuation plans, and running through what to do in a spill. Making this training routine, not just part of new employee orientation, makes a difference. Workers remember what steps keep them safe in the heat of a near-miss.
Employers who invest in equipment designed for chemical handling—ventilation systems, respirators for confined spaces, and continuous gas monitors—give teams an edge. These tools spot problems early and keep exposure in check. Prompt cleanup stations, proper storage, and real-time air quality checks cut down on guesswork about what’s floating in the air. Simple steps—like using spark-proof tools and checking storage seals—prevent accidents before someone ends up in the ER.
Addressing Gaps and Moving Forward
OSHA and NIOSH lay out clear exposure limits for isobutyl mercaptan, but on-site enforcement matters more than paper policies. People rely on signage and alarms to spot trouble, but nothing replaces open communication between crews and supervisors. Reporting near-misses, sharing knowledge about old leaks or breaks, and building a culture where health concerns aren’t brushed aside helps protect everyone. No one should feel rushed or pressured to work through symptoms just to hit a quota.
Good safety habits stack up—first as a shield against daily wear and tear, then as a safety net when something big goes wrong. Focusing on basic protective actions, equipment upkeep, and making health a priority keeps the smell of isobutyl mercaptan exactly where it belongs: as a warning, not as an emergency.
Chemistry at Street Level: What Is Isobutyl Mercaptan?
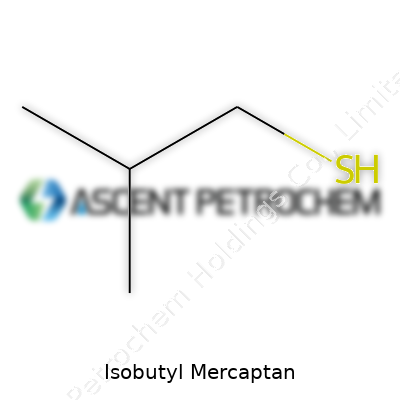
Isobutyl mercaptan carries a strong, memorable odor that seems to stick in a room long after a bottle gets closed. It’s one of those chemicals that keeps people in labs on their toes, but it goes well beyond academic curiosity. Its formula is C4H10S, which tells you that every molecule holds four carbon atoms, ten hydrogens, and one lonely sulfur.
Breaking Down the Formula
Why does the structure matter? For people who handle chemicals daily, the layout of every atom has practical consequences. The backbone of isobutyl mercaptan traces three carbons in a row, with one branching off the second carbon. The structure looks like this:
CH3-CH2-CH(SH)-CH3
That “SH” group, known as a thiol or mercaptan group, is what produces the classic skunky, pungent scent. The shape of the molecule also affects how it interacts with other substances. For those in the energy sector, this makes it valuable as a warning odorant for natural gas and propane. Without this, you’d never know a gas leak happened. That’s one practical way chemistry saves lives, in real time, on a real street.
The Reason for the Smell
Ask anyone who’s caught a whiff of mercaptans — it’s an unforgettable experience. That sulfur atom doesn’t just make the molecule unique; it changes how the molecule sits in the air and how it gets picked up by noses. Reports show that people can smell isobutyl mercaptan at concentrations as low as 0.0001 parts per million. No proprietary technology required; just a sharp nose. This kind of sensitivity has led to strict safety protocols in industries that transport or use combustible gases. The compound’s role as a built-in alert is far from optional. Without such chemical sentinels, tragedies from undetected leaks would multiply.
Controlling Exposure and Managing Risks
Anyone who spends time in a lab or plant environment knows the value of controlling strong-smelling chemicals. Besides solid ventilation, spill protocols must stay clear and practiced. Research published in occupational health journals recommends limiting exposure and using monitoring devices where isobutyl mercaptan gets stored or handled. It doesn’t just irritate the nose; overexposure brings headaches and nausea for workers. Ensuring everyone understands the reason behind the rotten-egg aroma in workspaces connects back to robust safety cultures.
Cleaner Solutions for Tomorrow
There’s a push for cleaner, safer alternatives in chemical signaling, but the fact remains: few substitutes work as well for odorizing fuel as mercaptans like isobutyl mercaptan. Engineering a replacement means finding something equally noticeable in the tiniest amounts, non-toxic, and affordable. Current research leans on advanced sensor technology for gas detection, which might eventually reduce reliance on traditional odorants. Still, the backbone of public safety owes a big debt to these smelly molecules for now.
The Bottom Line
Isobutyl mercaptan might never win any popularity contest for its scent, but its formula C4H10S and structural specifics keep it on the front lines of safety. It’s a reminder that sometimes chemistry’s practical side—smell included—delivers the protection people rarely realize they need.
Why Take Extra Care?
Anyone who’s ever dealt with Isobutyl Mercaptan knows its strong, skunky odor lingers and travels fast. Even a tiny spill can clear a room or spark complaints from the neighbors. The National Institute for Occupational Safety and Health (NIOSH) lists it as hazardous. Direct contact with skin or inhaling the vapors causes headaches, nausea, and respiratory issues. All of this at concentrations far below those found in a lab or plant environment. A mistake here isn’t just unpleasant—it’s a health issue and a headache for compliance down the line.
Having handled chemicals with scents that stick to clothes and hair well past the workday, I’ve learned that a sloppy setup makes for constant stress. One drop breaching a poorly sealed drum leaves you searching for air and reaching for the emergency phone. Getting storage and handling right keeps the building safe long-term, cuts down on unwanted emergencies, and keeps the professionals working nearby on good terms with their own noses.
Solid Storage Practices
Store Isobutyl Mercaptan in tightly sealed steel drums or containers built to resist organic compounds. Plastic or unlined containers break down or allow vapor leaks. The chemical attacks certain rubbers and plastics, so those drum seals need a compatibility check before anyone signs off. Pressure builds fast, especially during the summer months or in poorly ventilated sheds. That means leaving headspace in containers and sticking to shaded, temperature-controlled storage.
Ignition is always a risk. Isobutyl Mercaptan has a low flash point. A stray spark, static discharge, or cigarette end starts trouble. Any facility storing this compound should keep flames, welding work, or even phones out of the area. Good practice posts clear warning signs and trains everyone in safe entry and exit procedures. Grounded containers and anti-static measures give peace of mind during transfers. Past mistakes in chemical plants taught harsh lessons—skipping a ground wire is an expensive shortcut if you get unlucky.
Vapor Control and Spill Readiness
Ventilation must be mechanical—not just a small window cracked open. Vapors from Isobutyl Mercaptan hang low and accumulate. If buildup isn’t vented, just opening a drum can waft fumes through the whole space. Work happening above open drums, without a proper fume hood or extraction fan, sends strong vapor directly into lungs and workspaces.
Drip trays under drums minimize the reach of accidental spills. I have seen absorbent sand and spill kits save many hours of cleanup and worry. Spill response needs more than a mop—it needs ready supplies, clear procedures, and training to know when it’s time to call emergency teams. Workers should always have gloves and goggles made for chemical exposure, not generic ones. Given how quickly skin and eyes react, taking shortcuts here gets people hurt fast.
Transport Pointers
Transport only in approved, clearly labeled containers. Secure these against shifting, and never load them with food or personal items. All drivers carrying Isobutyl Mercaptan should carry up-to-date safety data sheets and instructions for spill response. Every misstep, whether a loose lid or dropped drum, has the potential for both a workplace health risk and a regulatory headache—and it’s always easier to do it right than fix it later on.
Understanding What’s on Your Hands
Isobutyl Mercaptan isn’t something you want leaking or mixing with everyday trash. If you’ve ever worked with odorant chemicals or managed a lab, you’ll know even a whiff can clear a room. Even aside from the stink, it’s hazardous for health, flammable, and can mess with soil and water if tossed the wrong way. I’ve seen folks try shortcuts, but environmental officers don’t mess around with chemicals this nasty. Cities have racked up fines over poor chemical handling and the headaches never end for those stuck cleaning up after a lazy disposal.
Why Proper Disposal Matters
I personally remember a fire department responding to a leak in an old factory’s storage room. Their report mentioned headaches, dizziness, and complaints from neighbors for days. The cleanup took a week, not just hours. The EPA tracks incidents like these to remind organizations that safety rules have teeth. Averting environmental problems starts with simple choices: good labeling, secure storage, and not waiting until it’s late to make a disposal plan.
What Real Solutions Look Like
1. Work with Licensed Chemical Waste Handlers
Throwing isobutyl mercaptan down a drain or into the trash isn’t just illegal — it poisons water and risks the sanity of anyone nearby. Professional hazardous waste handlers know the legal rules. They label drums, track movements, and provide paperwork. Even on a small scale, I call the waste number before moving a drop. Local governments publish lists of contractors and many offer pick-up for businesses and large users. The process isn’t fast, but traceability keeps everyone honest.
2. Don’t Mix Chemicals
I once witnessed a rookie pour leftovers into a nearly full drum without checking if the chemicals matched. Turns out, that can cause violent reactions. Isobutyl mercaptan does nasty things with oxidizers and acids. Never combine with unknown waste hoping to save space. Big companies mark every container and review waste logs regularly. In your own shop, keep separate drums for organics and avoid playing chemist.
3. Ventilate and Store Correctly Before Pickup
The stuff evaporates fast and lingers, so keep it in well-sealed containers. Place drums in a ventilated space, away from sparks or anything flammable. Store away from acids and sources of heat. These basics have stopped more than one near-miss at small research labs and maintenance areas. Employees handling the storage area deserve respirators and gloves. I’ve enforced that in any lab I supervised and had zero workplace incidents with mercaptans.
4. Paperwork Isn’t Just for Box Tickers
The legal side backs up your safety. Detailed manifests, logs of how much you’re discarding, and the contact info of the waste company build a solid record. Audits and spot checks happen, and being organized earns respect and avoids stress. Even tech startups, not just chemical firms, now keep digital logs. I’ve heard of regulators dropping in following local complaints, and only those with good records walked away worry-free.
A Little Effort Pays Off
In the end, disposing of isobutyl mercaptan responsibly solves more than just a storage problem. It keeps your neighbors happy, protects local streams, and avoids lawsuits. The hassle upfront saves pain later, both for your crew and for anyone living nearby. Rely on experienced handlers, never cut corners, and run a tight ship with records. Chemical safety isn’t glamorous, but doing it well shows real care for your team and your environment.

| Names | |
| Preferred IUPAC name | 2-Methylpropane-1-thiol |
| Other names |
1-Butylthiol
2-Methyl-1-propanethiol isobutanethiol |
| Pronunciation | /ˌaɪsəˈbjuːtɪl mɜːrˈkæptæn/ |
| Identifiers | |
| CAS Number | 513-44-0 |
| Beilstein Reference | 0956811 |
| ChEBI | CHEBI:28593 |
| ChEMBL | CHEMBL25216 |
| ChemSpider | 7929 |
| DrugBank | DB11665 |
| ECHA InfoCard | 10e4e73a-49e9-4b73-b607-7a9f3a6582a7 |
| EC Number | 200-268-9 |
| Gmelin Reference | 6036 |
| KEGG | C06164 |
| MeSH | D008134 |
| PubChem CID | 15606 |
| RTECS number | **NN8925000** |
| UNII | 3E77N19H3F |
| UN number | UN1993 |
| Properties | |
| Chemical formula | C4H10S |
| Molar mass | **90.19 g/mol** |
| Appearance | Colorless liquid with an unpleasant odor |
| Odor | unpleasant, strong, penetrating, cabbage-like |
| Density | 0.794 g/mL at 25 °C |
| Solubility in water | Insoluble |
| log P | 1.96 |
| Vapor pressure | 195 mmHg (20°C) |
| Acidity (pKa) | 10.8 |
| Basicity (pKb) | Isobutyl Mercaptan pKb = 3.77 |
| Magnetic susceptibility (χ) | -9.41×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.414 |
| Viscosity | 0.67 mPa·s (at 20°C) |
| Dipole moment | 1.66 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 242.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -109.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3895 kJ/mol |
| Pharmacology | |
| ATC code | V03AB36 |
| Hazards | |
| GHS labelling | GHS02, GHS06, GHS07, GHS08 |
| Pictograms | GHS02,GHS06,GHS07 |
| Signal word | Danger |
| Precautionary statements | P210, P261, P273, P280, P301+P310, P304+P340, P305+P351+P338, P311, P403+P235, P501 |
| NFPA 704 (fire diamond) | '2-4-0-☠' |
| Autoignition temperature | 250°C (482°F) |
| Explosive limits | 1.7% - 10.6% |
| Lethal dose or concentration | Lethal dose or concentration: "LD50 (oral, rat): 179 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 179 mg/kg |
| NIOSH | ME1400000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Isobutyl Mercaptan is "0.5 ppm (1.5 mg/m3) as an 8-hour TWA (OSHA)". |
| REL (Recommended) | Rel = "0.5 ppm (1.3 mg/m3) TWA |
| IDLH (Immediate danger) | 150 ppm |
| Related compounds | |
| Related compounds |
Methanethiol
Ethanethiol n-Propyl mercaptan Isopropyl mercaptan n-Butyl mercaptan tert-Butyl mercaptan |
