Phosphorus Pentasulfide: Its Journey, Uses, Safety, and Future
Historical Development
Phosphorus pentasulfide popped up at the tail end of the 19th century, when chemists first noticed how phosphorus and sulfur could combine in very distinct ratios. Early manufacturers hunted for better ways to produce matches and lubricants, but along the way, this bright yellow compound caught their eye—and for good reason. The age of industrial chemistry kept finding new possibilities for materials whose properties stood out from the crowd, and phosphorus pentasulfide didn’t disappoint. Through much trial and error, researchers moved from rough lab synthesis to industrial-scale production, paving the way for it to play a key part in the additives and lubricant industries. The chemical’s journey mirrors a wider shift in industry: going past basic science into fine-tuned large-scale manufacturing.
Product Overview
Commercial phosphorus pentasulfide forms as greenish-yellow crystals that carry a distinct, not-so-pleasant odor, reminiscent of rotten onions—an experience anyone working near bulk stores won’t forget. Industries prize the substance as a reliable building block for making additives that improve performance in lubricants, detergents, and insecticides. In trade and supply chains, it often arrives in steel drums or sacks, labeled for quick identification to avoid surprises during storage or handling.
Physical & Chemical Properties
The compound’s melting point sits around 288°C and it starts breaking down above 300°C, giving workers a clear warning that controlling temperature is a must. Unlike many common chemicals, phosphorus pentasulfide doesn’t dissolve in water. If you drop some in a beaker, you get a vigorous reaction instead, quickly releasing hydrogen sulfide gas. The substance weighs more than water (density 2.09 g/cm³) and looks waxy and solid at room temperature. Once exposed, its fumes sting the nose and eyes, so a well-ventilated space quickly becomes a top priority.
Technical Specifications & Labeling
In real-world trade, buyers expect a minimum purity around 99%, and sellers must document sulfur and phosphorus content. Most containers arrive with hazard stickers pointing out toxicity and flammability. From my work in procurement, import documentation never skips detailed hazard codes, so customs and handlers know exactly how to treat what’s inside. Product certificates tend to follow local standards set by organizations such as ASTM or ISO, with batch numbers, production dates, and shelf life included to limit mistakes down the line.
Preparation Method
Industrial synthesis depends on the direct union of red or white phosphorus with molten sulfur, inside a tightly sealed reactor to keep air and moisture out. Factories introduce phosphorus to an excess of liquid sulfur under nitrogen or some other inert blanket. Keeping air away prevents accidental fires. Operators closely control temperatures around 300°C, sometimes stirring the batch for uniform results. What comes out is cooled, drained, crushed, then bagged—every step overseen to keep batches consistent. Over years of plant visits, I’ve noticed major investments made to capture hydrogen sulfide gas, which not only cuts emissions but also protects workers from danger.
Chemical Reactions & Modifications
Phosphorus pentasulfide reacts with alcohols and phenols to produce phosphorothioate esters—crucial intermediates for oil additives, flame retardants, and pesticides. Mix it with water and you get a rapid, violent reaction, splitting off hydrogen sulfide and leaving behind a slimy, acidic residue. With bases or amines, scientists generate different phosphorus-sulfur compounds, each tailored for a unique role in stabilizing fuels, greasing gears, or protecting crops. Decades of research into its reactivity have carved out a toolbox for manufacturers who need fine control over product performance.
Synonyms & Product Names
On order forms, the material shows up under several names. Commonly, buyers see “Phosphorus(V) sulfide” or “P2S5,” and sometimes “pentasulfide of phosphorus.” Suppliers pushing branding offer versions called “Thionex” or “Luxan P2S5,” but these trademark names don't change the substance inside, just how it hits the market and shows up in regulatory paperwork.
Safety & Operational Standards
Factories and distribution centers set strict rules for handling phosphorus pentasulfide, not just because of its flammability but its tendency to release hydrogen sulfide, which can kill at high concentrations. Every shift begins with a reminder about personal protective equipment—gloves, goggles, and, in poorly ventilated spaces, proper respirators. Storage rooms run dry and cool with metal drums sealed tight; water leaks or sudden humidity can spark hazardous reactions. Those responsible for safety often point out emergency showers and exhaust fans within arm’s reach. Hazard communication doesn’t stop at workers: local firefighters tour plants to get familiar with layouts and response plans, especially after near-misses are reported and logged for future training.
Application Area
Phosphorus pentasulfide serves as a standout player in the world of lubricant additives. As a backbone for zinc dithiophosphates—key oil stabilizers—it keeps engines and gearboxes humming smoothly. Agrochemical formulations depend on it to manufacture insecticides that keep pests in check while supporting food supplies. In mining and flotation plants, technicians use phosphorus pentasulfide-based reagents to separate minerals efficiently from waste rock. Years in supply logistics have shown me factories in Asia, Europe, and North America all keep regular demand for these uses, with general manufacturing and consumer safety regulations shaping which applications thrive in each region.
Research & Development
Research labs keep looking for better ways to harness phosphorus pentasulfide. Chemists run pilot projects to design new oil additives that stretch engine lifespans and cut emissions. The push to find smarter, safer pesticides also leans on this material, with teams tweaking molecular structures to reduce environmental impact without losing effectiveness. A few universities publish papers on stabilizing its reactions under mild conditions, aiming to cut risks for workers and boost product yields. Having spoken with several R&D managers, it’s clear that the pressure for safer, more versatile materials continues to climb—and they see phosphorus pentasulfide as key to that charge.
Toxicity Research
Toxicologists don’t take phosphorus pentasulfide lightly. Short-term exposure irritates eyes, lungs, and skin; long-term inhalation of dust or fumes can do lasting harm. Hydrogen sulfide, which comes free during accidental spills or poor handling, ranks among the most feared industrial gases for plant safety engineers. Health agencies regularly update exposure limits and conduct animal tests to catch subtler symptoms or rare complications. Medical reports from exposed workers highlight headaches, dizziness, and chronic respiratory irritation as recurring issues—clear signals for the need to strengthen safety programs and personal monitoring.
Future Prospects
Clean technology and tighter regulations push phosphorus pentasulfide makers to rethink both production methods and end-uses. The shift toward electric vehicles doesn’t erase demand for top-tier lubricants—if anything, gearboxes and specialized fluids depend more than ever on advanced additives. New crops and pest species keep the agrochemical sector experimenting with formulations based on this material. Environmental regulations spur investment in recycling side-streams and locking up toxic gases before they leave factory gates. Based on recent trade shows and technical journals, the next decade will likely see phosphorus pentasulfide production adapt with greener synthesis, tighter emission controls, and a wider portfolio of specialty chemicals for industries facing stricter sustainability targets.
Phosphorus Pentasulfide in Industry and Daily Life
People rarely see phosphorus pentasulfide on store shelves, yet entire industries lean on this yellowish powder. Its uses stretch from lubricants to chemical production, but the real story comes down to its effect on products many of us depend on, even if most will never hear the name outside a chemistry class.
Key Ingredient in Making Lubricant Additives
Car engines run better and last longer thanks to modern engine oils. Behind the scenes, phosphorus pentasulfide acts as a building block for additives known as zinc dithiophosphates. These additives fight wear, reduce corrosion, and keep metal surface problems at bay. Without them, engines suffer more friction, break down much quicker, and the cost of repairs climbs. The average driver doesn’t think about engine chemistry, but smooth trips to work or vacation depend on compounds made possible with this substance.
Vital Link in the Pesticide Chain
Go past the city and farmers rely on phosphorus pentasulfide for crop protection. It helps create pesticides like parathion and malathion, which have boosted yields for years. These chemicals have allowed growers to produce more food with fewer losses from bugs and disease. There’s a flip side — mishandled pesticides mean environmental damage and health risks. Balance calls for education, oversight, and research into safer alternatives while recognizing the boost such chemicals provided, especially before the spread of advanced farming methods.
Role in Safety Matches and Fireworks
Before lighters, matches came first. Phosphorus pentasulfide helps create the material on match heads that lights easily. This property also finds its way into fireworks mixtures, giving that quick ignition many have admired every New Year’s Eve or festival. Every pop and sparkle at these events draws from a chemical reaction made possible by this compound. Most folks see only the celebration, but factory workers handle the ingredients directly, which reminds everyone that these industries must always focus on safety and worker protection.
From Mining to Detergents
Mining companies use chemicals made with phosphorus pentasulfide to separate minerals from ore. This technique, known as flotation, has improved the quality of raw materials feeding into everything from electronics to construction materials. In another shift, detergent makers have used related sulfurized compounds to develop better soaps and cleaners, although stricter rules around pollution have changed some industrial recipes. Seeing this pattern shows production chains are always in motion, responding to both new science and everyday needs.
Concerns and Safer Handling
Exposure to phosphorus pentasulfide itself can cause health problems, especially by inhalation. Its reaction with water or moisture forms hydrogen sulfide gas — a toxic compound. The record shows mishaps can occur where training and ventilation fall short. Factory workers and transport teams rely on well-designed equipment, strong rules, and regular checks. These efforts have improved over the years, proving that technology alone never replaces the human eye or common sense.
Looking Ahead
Chemicals like phosphorus pentasulfide play a part in the goods we trust daily, from keeping engines running to the food supply and even simple household matches. Greater transparency, more research into alternatives, and investment in safer production practices keep industry on a steady course. No single solution exists, but a blend of oversight, innovation, and respect for those at every link of the supply chain brings progress — and a good reminder about the quiet power behind many modern conveniences.
Getting to Know the Risk
Phosphorus pentasulfide pops up in more places than most people realize. Used in everything from lubricating oil additives to pesticides, its presence stretches across industries touching agriculture, manufacturing, and chemical research. The problem doesn’t lie only in its broad use, but in how its properties create risk for people and the environment.
Health Dangers: Not Just a Label
The hazards around phosphorus pentasulfide aren’t just regulatory jargon. Breathing in its dust or fumes causes burning in the throat, coughing, and sometimes lung irritation severe enough to land a worker in the hospital. Skin contact doesn’t go unnoticed, either—as little as a splash can bring about redness, itching, or more serious burns. If this stuff gets anywhere near your eyes, lasting damage can follow. Anyone who has handled this compound quickly learns respect for proper gloves, goggles, and dust masks.
One of the trickiest aspects involves what phosphorus pentasulfide does near water. When it reacts with moisture, it creates hydrogen sulfide gas. For folks who remember their high-school chemistry, that gas smells like rotten eggs and can be downright deadly at higher concentrations. Exposure to hydrogen sulfide even at lower levels brings on headaches, dizziness, and nausea. At high levels, it can overwhelm the senses and shut down breathing in minutes.
Environmental Risks Worth Noticing
I’ve walked past old storage sheds where small leaks have caused yellowish crusts to build up at the floor's edge. If rainwater seeps inside, phosphorus pentasulfide reacts vigorously and starts throwing hydrogen sulfide into the air. Imagine that at a bigger scale—near a river or in a factory drain. It threatens fish, wildlife, and in some regions, entire communities that depend on these waterways.
Folks living around manufacturing plants often worry about accidental releases, especially on stormy days. A spill rapidly turns into something far more dangerous as soon as it touches puddles or even damp soil. Cleanup is not simple, either—removing contaminated earth means heavy equipment and a big disposal bill. Prevention feels much more appealing than picking up the pieces later.
What Real Solutions Look Like
Pushing regulations alone doesn’t cut it. Workers need real training beyond reading a safety sheet. I’ve seen a chemical plant leader run hands-on drills where everyone knows where respirators are, and nobody just assumes the next person will handle a spill. That sort of culture shift matters.
Companies with a good track record usually go beyond minimum requirements. They use sealed containers, keep a tight lid on storage and transport, and have sensors to catch hydrogen sulfide quickly. Community groups sometimes work with plant operators to double-check safety procedures or call for more transparency around incidents. These strategies build trust and save lives.
The Bottom Line
Phosphorus pentasulfide creates real hazards—certain situations can turn it from useful chemical to dangerous threat. The key is vigilance, from the shop floor to management, all the way up to local emergency response teams. Protecting workers and the planet means taking the risks seriously and using every tool to keep this compound under control. My experience tells me, every extra bit of care helps.
What’s at Stake with Phosphorus Pentasulfide?
Phosphorus pentasulfide doesn’t ask for attention until it winds up mixed with the wrong stuff or sits in a leaky container. Plenty remember their first lesson with chemicals like this: skip a step, things can get complicated. This yellow-green solid is more than just an industrial staple for lubricants and pesticides—it carries real risks. It fumes up in moist air, produces hydrogen sulfide gas, and catches fire if things get sloppy. Management mistakes can turn any warehouse into a hazmat story.
Ventilation Keeps Trouble Outside
My time around warehouses proved long ago that cramped, musty storage spaces leave no margin for error. Phosphorus pentasulfide needs a place where fresh air keeps fumes from building up. Workers appreciate a dry, cool, well-aired environment—so do those bags and drums. It’s not enough to stash it anywhere out of the rain; high humidity brings out its most toxic qualities.
Nothing Beats a Solid Sealed Drum
Those who work with bulk chemicals know the importance of a tightly sealed container. Once, in a facility without reliable covers, the cost of “just for today” thinking soared—damaged barrels meant ruined product and a scramble as gas alarms blared. With phosphorus pentasulfide, a simple tight seal wins every time. Metal drums with lined lids block air and moisture, save on loss, and keep that rotten egg smell inside.
Fire Hazards Deserve Respect
Firefighters often point out how easily some chemicals catch fire, and phosphorus pentasulfide belongs on that list. Sparks, static charges, or a carelessly tossed cigarette can start a blaze that’s hard to handle. Separation from flammable liquids and oxidizers keeps things safer. Sand buckets, Class D extinguishers, and clear evacuation routes never seem extravagant once an emergency starts. Folks in the industry tend to swap stories about near misses, and the best ones are the ones avoided with basic tools and habits.
Training Makes the Difference
Even seasoned workers benefit from a run-through now and then. One shift without reminders, and shortcuts creep in. Everyone knows not to open drums unless they’ve got gloves and face protection, but fatigue tests good habits. Regular discussions, visible safety signs, and honest talk about previous incidents shape a responsible culture. Emergency showers and eyewash stations within reach might sound like overkill—until someone needs them.
Spill Response Comes Down to Speed
Spills spread fast and create toxic vapors when water shows up. Shovels, absorbent materials, and ventilated cleanup gear minimize harm, but only if workers move quickly. Every second counts. No one wants emergency crews arriving to a bigger mess because a team froze up or gear sat on a high shelf. Quick response plans prove their worth in those moments.
Why This Matters Now
Growing demand in agriculture, mining, and manufacturing means phosphorus pentasulfide isn’t going anywhere. Safe handling protects workers, businesses, and nearby communities. Storage rules and responsible habits don’t just tick boxes—they help keep everyone out of the headlines for all the wrong reasons. Getting the basics right each day can prevent years of regret.
The Basics of Phosphorus Pentasulfide
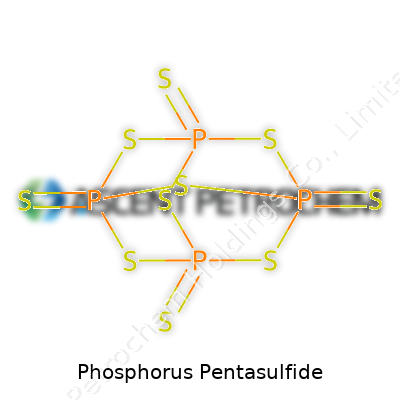
The chemical formula for phosphorus pentasulfide is P2S5. This yellowish solid often pops up in industrial conversations about matches, lubricants, and specialty chemicals. With two phosphorus atoms joined to five sulfur atoms, the structure gives it a unique set of properties, shaping both its opportunities and the safety concerns it comes with.
Why Phosphorus Pentasulfide Gets Attention
Phosphorus pentasulfide often gets its claim to fame in the world of lubricants, specifically the ones used in high-stress machinery. Gear oils and cutting fluids count on compounds made from it to reduce friction and avoid wear. Farmers also run across it due to its role in making pesticides. With phosphorus and sulfur being key nutrients for plants, it’s not surprising that chemists found ways to use them together in crop protection.
Those who work with it up close talk about its strong odor—a sharp, garlicky smell that signals more than just discomfort. Under the wrong conditions, this chemical can release hydrogen sulfide, which poses its own risks. The way it reacts with water stands out most: the reaction can produce toxic gases and even lead to fires if not handled right. That’s why safety folk put so much emphasis on training and personal protection in shops and labs using it.
Safety and Handling Challenges
I remember visiting an old factory where operators used this material for match production. The amount of dust control, specialized storage, and ventilation was something to see. Heat, moisture, and open flames had no place there—not just as a rule, but as a way of working shaped by years of hard lessons. Many accidents in chemical plants trace back to a single missed warning or a shortcut, especially where moisture finds its way in.
Phosphorus pentasulfide demands well-maintained containers and clear procedures. Any spill gets treated not just as mess but as potential disaster due to the risk of toxic gas. Fire crews responding to incidents with this material don’t rely on water. Instead, they use sand or dry chemicals, knowing water makes matters worse.
Built on Research and Regulation
The safety data behind this chemical runs deep. Chemists and regulatory agencies—like OSHA and the EPA—have years of studies looking at its health effects, environmental impact, and necessary exposure limits. Industry groups collaborate on best practices, putting those findings into the hands of workers through training programs and updated guidelines. That approach gives people the tools to protect themselves and the environment.
Looking for Safer Alternatives
Some companies try shifting away from phosphorus pentasulfide, either by updating processes or switching to less hazardous chemicals. Change like this comes from demands for safer workplaces, stricter waste controls, and pressure from communities living near chemical plants. Researchers keep testing new ways to achieve the same lubrication and crop protection without the same risks.
Phosphorus Pentasulfide’s Role Moving Forward
Anyone curious about this compound’s place in daily life finds it in everything from automotive parts to fields of crops. Balancing industrial needs with health concerns takes more than memorizing a formula. It calls for respect, vigilance, and constant learning—grounded in both lab results and hard-won experience. Phosphorus pentasulfide, P2S5, stands as a reminder that chemistry’s benefits and dangers often come bound together.
Why Handling Matters
Phosphorus pentasulfide has a long history in chemical production. It’s a building block for pesticides, lubricants, and matches, but even small mistakes can lead to burns, fires, or toxic gas exposure. Many folks have witnessed something as simple as a rushed job kicking off a dangerous chain reaction. Staying attentive pays off not only for personal safety, but for everyone sharing the space.
The Hazards You Face
This chemical reacts fiercely with water and even damp air, giving off highly toxic and flammable gases like hydrogen sulfide. Any leak, spill, or puff of dust can bring risk. I remember working near a storage room where a small spill went unnoticed. The rotten egg smell tipped off our crew, and a quick evacuation avoided a bigger problem. It sticks with you.
The Gear That Keeps You Safe
Gloves matter, but not every pair stands up to this chemical. Neoprene or rubber gloves shield hands, along with chemical splash goggles. Face shields add a layer of protection against stinging splashes and unexpected sprays. Long sleeves and pants aren’t just old habits; clothes lined with flame-resistant fibers help when dealing with reactive powders. Folks working in dusty settings often rely on full-face respirators or supplied-air systems to keep fumes and dust from reaching the lungs.
Storage and Handling Routines
Storing phosphorus pentasulfide calls for closed, dry containers, away from anything that could spark or drip moisture. I’ve seen makeshift storage rooms lead to more headaches than they solved. Dedicated shelving, spill trays, and real separation from acids or oxidizers keep accidents off the daily agenda. Only trained crew should have access. Training shouldn’t just be about reading a binder – it’s walking through real scenarios together, so people know how to react.
The Role of Ventilation and Monitors
Spending long hours in a poorly ventilated room leaves no margin for error. Local exhaust, like fume hoods or point-source fans, draws away any vapor before it spreads. Gas detectors and hydrogen sulfide alarms give early warnings. Regular checks, not just yearly ones, mean the safety gear works when things get tense.
Steps for Small Spills or Fires
Nobody likes finding a spill, but pretending it won’t happen sets up a bigger emergency. Teams familiar with their emergency gear move quickly to contain and clean using shovels, scoops, and dry sand—never water. For fires, the instinct sometimes calls for water, but class D fire extinguishers make a difference. Evacuating the space and calling in trained responders keeps a bad day from turning tragic.
Training and Communication
I’ve noticed that the best safety records go hand-in-hand with strong communication. If someone spots an issue, they speak up. New hires shadow veterans, soaking up not just the rules, but the unwritten tricks. Regular refreshers and walk-through drills root good habits. Simple practices, like sharing what went wrong in past incidents, set a tone that puts people first.

| Names | |
| Preferred IUPAC name | tetraphosphorus decasulfide |
| Other names |
Tetraphosphorus decasulfide
Phosphorus(V) sulfide Phosphorus pentasulphide |
| Pronunciation | /ˌfɒs.fə.rəs ˌpɛn.təˈsʌl.faɪd/ |
| Identifiers | |
| CAS Number | 1314-80-3 |
| Beilstein Reference | 1713795 |
| ChEBI | CHEBI:48878 |
| ChEMBL | CHEMBL1565013 |
| ChemSpider | 21142776 |
| DrugBank | DB11190 |
| ECHA InfoCard | ECHA InfoCard: 100.031.954 |
| EC Number | 215-242-4 |
| Gmelin Reference | 52744 |
| KEGG | C07414 |
| MeSH | D010754 |
| PubChem CID | 24414 |
| RTECS number | TH4375000 |
| UNII | Q4347K127T |
| UN number | UN1340 |
| CompTox Dashboard (EPA) | DTXSID2021777 |
| Properties | |
| Chemical formula | P2S5 |
| Molar mass | 222.27 g/mol |
| Appearance | Yellow crystals. |
| Odor | rotten eggs |
| Density | 2.09 g/cm³ |
| Solubility in water | insoluble |
| log P | -0.21 |
| Vapor pressure | 0.0227 mmHg (25°C) |
| Magnetic susceptibility (χ) | -48.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.627 |
| Viscosity | Viscous solid |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 178.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -308.9 kJ/mol |
| Pharmacology | |
| ATC code | V09XX04 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07, GHS08 |
| Signal word | Danger |
| Hazard statements | H261, H301, H314, H400 |
| Precautionary statements | P210, P261, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P370+P378, P402+P404, P501 |
| NFPA 704 (fire diamond) | 2-4-3-W |
| Flash point | 42 °C (108 °F; 315 K) |
| Autoignition temperature | 100 °C (212 °F; 373 K) |
| Lethal dose or concentration | LD50 oral rat 640 mg/kg |
| LD50 (median dose) | > Oral rat LD50: 300 mg/kg |
| NIOSH | **WF3490000** |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Phosphorus Pentasulfide: "1 mg/m³ (as phosphorus pentasulfide), 8-hour TWA (OSHA) |
| REL (Recommended) | 1 mg/m3 |
| IDLH (Immediate danger) | 100 mg/m3 |
| Related compounds | |
| Related compounds |
Thiophosphoryl chloride
Phosphorothioic acid Phosphorus trisulfide Phosphorus trichloride Phosphoric acid |
