Looking Deeper into Thiodiglycolic Acid: A Down-to-Earth Commentary
Historical Roots and the Journey of Discovery
Thiodiglycolic acid didn't pop out of nowhere. Its origins reach back to the wave of scientific breakthroughs in the early and mid-20th century, when chemists kept piecing together new sulfur-containing molecules. The drive to build better products—things like dyes, polymers, and drugs—pushed labs to experiment with ever more complex acids. Thiodiglycolic acid earned a spot in the chemical world as researchers explored new approaches to synthesis and industrial chemistry. As the decades rolled by, industries realized its potential, building demand on the back of rising plastics manufacturing and pharmaceutical research. Looking at this trajectory, it's clear how much scientific curiosity and economic need keep pushing boundaries in chemical production.
Peeling Back the Layers: What Makes Thiodiglycolic Acid Stand Out
Thiodiglycolic acid, also known by names like 2,2'-Thiodiacetic acid or TDGA, combines water solubility with the kind of reactivity that excites chemists. Its structure—marked by a central sulfur atom linking two carboxylic acid groups—leads to a mix of strong acidity and sulfur-based behavior. Unlike simpler acids, TDGA easily forms complexes with metal ions, showing up with unexpected colors and reactions in the lab. You won’t catch a whiff of a floral scent; instead, a slightly pungent, sharp fragrance signals the sulfur. White or pale crystals form at room temperature, dissolving smoothly in warm water or alcohol. Chemical manufacturers and lab technicians look for these physical and chemical traits because they allow TDGA to play different roles, from a synthetic building block to a soil remediation tool.
Digging Into the Specs: Technical Details and Labeling Practices
Professional settings demand concrete information. Details like the melting point—hovering around 107°C—for TDGA act as key checkpoints for purity and handling in industrial labs. Specifications from top chemical suppliers often call for a purity of 98% or higher, watched closely by quality control departments. Labeling follows strict regional and international guidelines. Each container displays the proper CAS number (123-93-3), along with recognized synonyms and hazard details. Safety Data Sheets require plain labeling: chemical identity, hazards, recommended storage, and emergency instructions go front and center to minimize risk and ensure smooth handoffs throughout the supply chain.
Preparation on the Ground: Real-World Synthesis Approaches
Many of today’s chemical producers stick to the classic approach—reacting dichloroethane with sodium thiosulfate under controlled temperature and acidity. This process yields sodium thiodiglycolate, which then gets acidified to produce thiodiglycolic acid itself. Reliability and scalability make this method a staple in commercial chemistry. Over the years, improvements trimmed reaction times and waste, yet batch processing remains common because it’s easier to control impurities and respond to changing market demands. Some research, especially for specialty applications, experiments with alternate feeds and catalysts to crank up yields or cut down environmental fallout.
A Closer Look at Chemical Reactions and Versatility
TDGA steps up in redox and condensation reactions. Sulfur grants opportunities for creating novel organosulfur compounds—powerful tools in drug discovery and metal extraction. Carboxyl groups link up with metals or organic fragments, which opens doors for its use in synthesizing chelating agents. In the field, those handling waste treatment rely on these interactions: thiodiglycolic acid forms stable complexes with heavy metals, helping pull contaminants from industrial discharge. Researchers plug TDGA into experiments exploring photochemistry and catalysis, investing hopes in new reaction routes that traditional acids can’t match. Plenty of these reactions tie into hybrid materials or high-performance coatings, fields that feed directly into green tech and new energy strategies.
Names and Nicknames: Synonyms and Trade Varieties
Products rarely go by a single name. Thiodiglycolic acid crops up in catalogs as 2,2'-Thiodiacetic acid, TDGA, and even by less formal variants shaped by manufacturing histories. Some suppliers use product codes or registered trademarks to stand out in a crowded space. Researchers and engineers switching between research papers, catalogs, and regulatory filings need to double-check these synonyms to dodge mix-ups—especially where safety, shipping, and international compliance cross paths. These naming conventions don’t just follow tradition; they shape how the material is tracked from factory to laboratory to end user.
Working Safely: Operational Standards and Hazards
Every chemical comes with a rulebook. Even compounds like thiodiglycolic acid—generally considered low-risk compared to harder-hitting toxins—deserve a close look at operational safety. Contact with skin or eyes still triggers irritation. In large quantities, its acidic and sulfur-containing nature poses risks through inhalation or spillage. Modern labs set up ventilation, mandate gloves, and post detailed storage instructions to keep spills and accidental exposures at bay. Emergency showers, eyewash stations, and clear labeling cut down on mistakes and injuries. Regulations from OSHA, REACH, and similar agencies push manufacturers and handlers to close compliance gaps and back up safety claims with regular training and audits. This culture matters—no lab wants a small mistake to snowball into a major health or environmental crisis.
Where TDGA Steps In: Real-World Applications
You find TDGA in places not often noticed by the public. It acts as a builder molecule in the production of chemicals, dyes, and some rubber additives. Its affinity for metals makes it a quiet workhorse in detoxifying industrial waste streams, helping clean up heavy-metal pollution before it sees a river or landfill. In pharmaceuticals, certain drug syntheses require sulfur bridges that TDGA helps construct, making medicines more stable or active. Analytical chemists use it to track chemical changes or to pull out trace elements from samples. Fields such as agriculture, battery research, and plastics take advantage of thiodiglycolic acid because it plugs into improvement projects across environmental safety, new product development, and cleaner production cycles.
Pushing the Envelope: Research and Development on TDGA
University and industry labs point their microscopes and reactors at TDGA, aiming to coax new value from an old compound. Some pursue greener methods—using renewable feedstocks or designing catalysts that don’t rely on rare or hazardous metals. Advanced materials scientists ask whether TDGA-derived polymers can outperform old standards, answering market pushes for longer-lasting, lighter, or safer products. In environmental research, scientists probe just how effective TDGA can be at remediating soils tainted by heavy metals or persistent industrial waste. It keeps coming up in conference proceedings and patents because industries crave solutions that make processes cheaper, safer, and less polluting.
The Question of Safety: Digging Into Toxicity Studies
No chemical escapes scrutiny. Animal studies and occupational health reports put thiodiglycolic acid under the environmental and public health microscope. Compared to many organosulfur compounds, TDGA shows up as less hazardous—acute toxicity levels are low, and most workplaces manage routine exposure with gloves and eye protection. Still, chronic studies demand attention. Researchers have flagged potential risks from long-term, high-concentration exposure, especially concerning kidney or liver function in animal models. The environmental fate of TDGA—how it moves through water, soil, and air, and how microbes break it down—sparks ongoing investigation. Timely updates to safety data sheets, along with government-mandated reporting, keep workers in the loop and build the trust that powers safe innovation in any lab or plant.
The Road Ahead: Where Thiodiglycolic Acid Might Go Next
If past experience predicts anything, thiodiglycolic acid has plenty of room to grow. Environmental cleanup remains a pressing concern, with heavy metals plaguing old industrial sites. New processing techniques and stricter regulations may force companies to seek out more effective or lower-cost chelating agents, giving TDGA a bigger platform. Sustainability trends push research into how this molecule can be synthesized with less waste and lower energy. Hybrid batteries and advanced composites beckon, where TDGA-derived compounds could boost everything from charge capacity to corrosion resistance. It may not make headlines, but the quiet workhorse qualities of thiodiglycolic acid show how a deep knowledge of chemistry shapes the tools for tackling some of industry’s most demanding problems.
What Thiodiglycolic Acid Does in Industry
Most people haven’t heard of thiodiglycolic acid, but many live in a world touched by its invisible hand. Chemists value it as a building block, especially when making things like dyes and plastics. In the textile field, the acid finds its way into certain dye processes because it helps create brighter, more lasting fabrics. PVC, a plastic that turns up in pipes and flooring, also depends on it for flexibility and workability.
Those who tinker with the structure of pesticides and rubber grab this acid for its ability to link molecules in just the right way. Its knack for binding sulfur comes in handy for vulcanizing rubber—a process that gives car tires toughness and bounce. Workers in fragrance and cleaner manufacturing might cross paths with traces of it, especially when synthesizing compounds that go into everyday lotions and sprays.
Health and Environmental Factors
Despite its usefulness, thiodiglycolic acid can stir up concerns. I’ve seen how factories occasionally shrug off best safety practices because they’re in a rush or understaffed. Direct contact with the pure stuff may irritate the skin, and inhalation could bother the lungs. Scientific reviews highlight how long-term exposure presents risks, reinforcing the need for strong protective gear and proper air handling. In laboratories, hardworking researchers have relied on fume hoods and gloves for years, but even then, accidents still happen.
Wastewater released during manufacturing sometimes carries traces of this acid. Aquatic life doesn’t take kindly to it in large quantities; studies show fish can suffer if water pollution goes unchecked. Neighbors living downwind from chemical plants worry about odors and unknown long-term impacts. Regulators in countries with heavy industry have started tightening water discharge rules, reflecting growing public unease.
Better Practices and Solutions
To lessen risks, companies can install better filtration systems and switch to closed-loop manufacturing so less waste escapes. As an operator, I’ve watched newer facilities invest in digital sensors. These tiny monitors pick up leaks early, giving crews the chance to fix problems before they grow. Older plants running on outdated designs face a bigger challenge, but government grants help them upgrade faster.
Research teams have studied using safer alternatives, but making the switch isn’t always simple. It takes money and teamwork from both industry and regulators. University chemists are testing greener synthesis methods, showing promise for reducing hazardous byproducts without killing off efficiency. Trade groups offer safety workshops and resources to help keep workers informed and routines sharp.
The Road Ahead
Trust matters as much as science here. Companies that stay open about their handling of acids and show they’re listening to local communities earn support. If everyday people better understand why thiodiglycolic acid matters—along with the risks—they can call for smarter policies and work toward safer workplaces. That’s how the conversation moves forward, from chemical jargon to shared responsibility.
Examining Real Risks
Thiodiglycolic acid often shows up in conversations among chemists and those concerned about industrial pollution. Found as a breakdown product of certain solvents, it can sneak into water near factories or show up in the urine of workers handling chemicals like vinyl chloride, a chemical tied to manufacturing plastics. Concerns about exposure go far beyond laboratory talk. This isn’t just a problem for scientists and plant workers—health effects can stretch into communities that neighbor industrial sites.
Scientific Findings on Health Impact
Researchers studying thiodiglycolic acid have observed its presence in humans mostly through urine samples. Workers exposed to chemicals like vinyl chloride tend to show higher levels of thiodiglycolic acid. Vinyl chloride has a record for causing cancer, and thiodiglycolic acid forms as the body tries to process it. Animal studies indicate high doses of thiodiglycolic acid can damage kidneys and might affect the nervous system, but direct studies in people remain limited. Still, science often points to metabolites like thiodiglycolic acid as red flags for tracking chemical exposure and potential illness.
Concerns for Industrial Communities
Communities living near plastic factories or chemical plants have real reasons to pay attention. More than just an abstract chemical, thiodiglycolic acid shows up as a sign that toxic exposure is happening. Over the years, public health agencies have studied areas hit hardest by industrial discharge and seen links between exposure markers and health problems. People living close to plants have higher chances of tracing these compounds in their blood or urine, which sends a message about environmental injustice and the need for better protection.
My Take on Its Importance
As someone who grew up in a region marked by chemical plants and spent years volunteering in environmental health outreach, concern over thiodiglycolic acid hits home. I’ve seen neighbors worry about kids playing outside, parents asking if local water is safe. No parent enjoys wondering if invisible substances in the air or water are placing their family at risk. Most people don’t walk around reading chemical safety reports, so the responsibility rests with industry leaders and local officials to keep the air and water clean. Tracking metabolites like thiodiglycolic acid can make a difference in catching problems before folks see illness in their homes.
Paths Forward
Communities can benefit when researchers test for chemicals like thiodiglycolic acid in local environments and public health surveys. Industrial plants shouldn’t brush off calls for monitoring or downplay results just because thiodiglycolic acid itself isn’t a headline toxin. Families deserve regular updates on testing near their homes. The EPA and similar agencies need consistent guidelines on what safe exposure looks like and should press for cleaner processing methods. Worker protections, such as improved ventilation, safer gear, and regular health checks, increase safety for those clocking in at chemical sites every day.
Transparency and Prevention Matter
Sharing research with those potentially affected, holding companies accountable for leaks, and supporting studies focused on community health can stop small issues from growing out of control. Thiodiglycolic acid is more than a lab result; it’s an early warning system that shouldn’t go ignored. By keeping the issue in the public eye and pressing for both transparency and prevention, vulnerable communities and workers stand a better chance of staying healthy.
Practical Considerations for Storage
Anyone who stores chemicals for work knows not all liquids behave the same way, and thiodiglycolic acid lands squarely in the category of “treat with care.” I’ve seen storage rooms where labels get ignored, lids are left crooked, and things go sideways fast. Thiodiglycolic acid asks for something better. Storing this acid in tightly-sealed, corrosion-resistant containers matters, usually polyethylene or glass. Those rusty metal drums in a forgotten warehouse corner cause bigger problems here–moisture and air speed up corrosion, sometimes giving off sulfur-like smells and leaving a sticky mess.
Keep thiodiglycolic acid containers away from direct sunlight and heat sources. Thermal cycles don’t just weaken seals but also promote unwanted reactions, particularly if trace metals get involved. If you ever worked a shift where a chemical started venting fumes unexpectedly, you remember the chaos – so storing the acid somewhere cool and shaded always beats cleaning sticky residue off a shelf.
Moisture creeps into most warehouses, especially during humid summers. Thiodiglycolic acid pulls in water from the air, leading to dilution and even by-products if left unchecked. On a busy day, staff might forget to immediately reseal a container, so training and checklists help. My experience says set up a culture where even the busiest worker double-checks lids before walking away.
Safe Handling in the Workplace
Anyone handling thiodiglycolic acid should suit up. Gloves that stand up to corrosives, goggles that block splashes, and long sleeves keep problems at bay. Acids seem simple until they hit an open cut or sensitive skin. Splashes aren’t rare either; pour one wrong, and the splash runs down a glove or bounces onto a forearm. I had a coworker ignore eye protection once, and the emergency eyewash station became their new best friend.
Good practice means using the acid in a well-ventilated space or a fume hood. Some technicians take shortcuts in small labs with poor airflow; this is a mistake that can turn a manageable chemical into an air-quality headache. Old ducts and broken fans don’t cut it, so regular equipment checks—like a working fume hood—should stay on every manager’s radar.
Chemical compatibility plays a big role. Thiodiglycolic acid reacts badly with oxidizers, alkalis, and strong bases, so mixing or storing near bleach, peroxide, or industrial cleaners risks dangerous reactions. Label containers with clear warnings and keep an updated chemical inventory. Over the years, I’ve seen plenty of close calls because someone stored incompatible acids on the same shelf as oxidizers.
In case of spillage, neutralize with sodium bicarbonate and mop up immediately while wearing full protective gear. Absorbent pads work better than shop paper towels, which quickly degrade. All waste needs clear labeling for the hazardous waste stream; dumping in a regular trash can or sink isn’t just wrong, it can trigger legal trouble and fines.
Solutions and Accountability
Preventing accidents with thiodiglycolic acid depends on rigorous training. Workers need regular refreshers on chemical hazards. Visual guides posted near storage areas, color-coded labels on shelves, and strict enforcement of locking rules help a lot. Electronic inventory systems cut down on misplaced containers, so a quick scan shows what’s on hand and what’s missing. Leadership can’t punt safety talks or let hazard labels fade. Someone has to own the process, checking, logging, and quickly responding to anything out of the ordinary.
Involving teams in safety audits opens eyes to gaps a supervisor might not catch. I’ve seen workplace cultures change for the better once teams bought into regular walkthroughs. A few dents in storage cabinets or mystery stains near containers should trigger fixes, not get shrugged at. With the right routines and accountability, storing and handling thiodiglycolic acid becomes part of safe, smart chemical work—not a daily gamble.
Breaking Down the Makeup
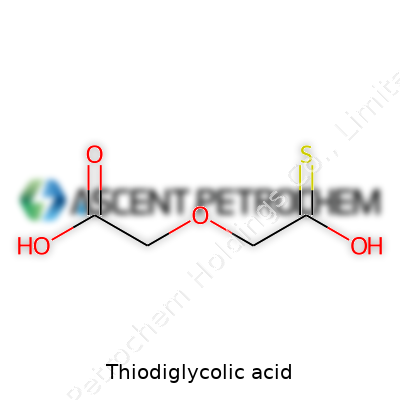
Thiodiglycolic acid comes with the chemical formula C4H8O4S. Its structure features a sulfur atom bridging two acetic acid groups. The way the atoms connect gives it a straightforward pattern: HOOC-CH2-S-CH2-COOH. The central sulfur atom is key, acting like glue between two carbon chains that both end in carboxylic acid groups.
The arrangement may sound simple, but this sulfur link turns plain acetic acid into something unique. Each molecule includes two carboxylic acid ends, giving it strong acidic properties and the ability to dissolve well in water. The sulfur is not just a background character. Chemists see this bridge as a channel for unique chemical behaviors, different from what you'd see if it was just oxygen in play.
Molecular Weight: Numbers Matter
Molecular weight shapes nearly every practical question about a compound. Thiodiglycolic acid carries a molar mass of 168.17 grams per mole. The calculation stacks up like this: four carbons (4 × 12.01), eight hydrogens (8 × 1.008), four oxygens (4 × 16.00), and one sulfur atom (32.07). Add those up, and you land at the molar mass many reference books quote.
For someone blending chemicals in the lab, this number means more than just a spot on a chart. It tells you the right measurements when mixing solutions or running reactions. A mistake on this figure could throw off an experiment, something I learned early on from a sudden change in pH and a wasted afternoon in a university lab. Precise molar mass keeps things moving and helps someone avoid costly errors, especially in processes that scale up from glass flasks to drums.
Why Structure Drives Use
I’ve noticed researchers and industrial chemists hunt for molecules with just the right structure. Thiodiglycolic acid’s shape opens doors. Its double-acid groups mean you can hook it onto other molecules or build polymers from it, and the sulfur boss in the middle creates traits that show up in rubber and plastics. Specialists watch the way it reacts—not every acid handles heat or the presence of metals the same way, but here, the sulfur brings fresh reactivity. This goes beyond textbooks; it plays into the research pushing for cleaner, more efficient chemical routes.
There’s a practical edge too. Thiodiglycolic acid winds up a marker in biological samples for workers exposed to chemicals like vinyl chloride. Doctors and toxicologists pick it out as a sign someone’s body has been working through tough exposures at the job. This little molecule pops up in urine as a warning flag, helping keep tabs on worker health in chemical manufacturing.
Paths Forward
Digging into the chemistry of molecules like thiodiglycolic acid, it’s easy to see why accuracy and transparency matter. More research around its breakdown could help reduce risk for people in factories, and make the process cleaner for communities that neighbor those plants. Sharing clear formulas, molar masses, and honest safety data helps both industry and ordinary people stay better informed. The next steps? Continued analysis of its health impacts and practical innovation in safer, greener synthesis. That’s progress grounded in scientific detail and lived experience.
Understanding Thiodiglycolic Acid in Everyday Use
Thiodiglycolic acid shows up in places you might not expect. This chemical comes out during the manufacturing of certain plastics, in the breakdown of specific pesticides, and sometimes in chemical labs working with solvents and dyes. With this level of use, it becomes part of the waste stream more often than most of us realize.
How Thiodiglycolic Acid Behaves in Nature
Drop thiodiglycolic acid into the environment and it doesn’t just disappear. It travels with water, moving through soil into groundwater or runoff into streams. Because it dissolves easily, monitoring shows it turning up near sites dealing with waste, pesticides, or manufacturing processes. This means farmers, rural communities, and anyone with a well become stakeholders in the discussion, even if they’ve never heard the name before.
What Science Says About Toxicity
Studies in labs show thiodiglycolic acid can impact living organisms. Researchers exposed aquatic animals, like water fleas, to the acid and saw reduced growth and survival rates. Fish also respond negatively, showing changes in enzyme levels and sometimes struggling with reproduction. These effects seem to happen at concentrations above typical background levels, but not every environment sees the same conditions.
Humans absorb thiodiglycolic acid as a metabolite after exposure to specific solvents. Low-level exposure usually gets cleared quickly by the kidneys, but lab workers dealing with larger amounts through skin contact or inhalation may face kidney strain and higher risks if safety procedures slip. From personal experience, labs take chemical handling seriously after a close call with a leaky container showed how quickly accidents compound. Without proper waste disposal and training, thiodiglycolic acid builds up in places it shouldn’t be.
Why Wastewater Treatment Matters
Municipal water treatment plants filter out a lot of pollutants, but thiodiglycolic acid’s high solubility lets it slip through traditional systems without much trouble. Facilities using advanced oxidation or activated carbon catch more of these chemicals, though few communities run such advanced setups. This leaves rural and small-town water systems especially vulnerable. Without modern treatment, even a small leak or illegal dumping makes a difference.
Solutions Rooted in Precaution and Innovation
Routine testing serves as a frontline defense. Communities living near chemical plants or agricultural runoff zones push local leaders to invest in better monitoring. Regular checks help catch problems before they reach a crisis point. Manufacturers also have a part in rethinking their process—capturing and reusing waste cuts down both raw material costs and environmental risks. This creates a bigger incentive for companies to look for greener pathways.
Switching to biodegradable alternatives or changing up which solvents and pesticides get used puts less strain on the environment. Education campaigns in local industries have real impacts; when teams know how to store, handle, and dispose of this chemical, spills drop and water sources stay cleaner. For researchers and policy-makers, sharing the latest toxicity data builds trust with the public and creates pressure for tougher standards.
Everyday Choices and Shared Responsibility
The story of thiodiglycolic acid isn’t unique—many chemicals follow this pattern. But focusing on prevention, transparency, and technology gives communities tools to stay ahead of contamination. Anyone with a stake in clean water gets a seat at the table, and that kind of shared vigilance can make all the difference in whether a chemical becomes a problem or stays under control.
| Names | |
| Preferred IUPAC name | 2,2′-Thiodiacetic acid |
| Other names |
TDGA
Thiodiglycollic acid Mercaptoacetic acid Dithioglycolic acid |
| Pronunciation | /ˌθaɪ.oʊ.daɪ.ɡlaɪˈkɒl.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 123-93-3 |
| Beilstein Reference | 1718730 |
| ChEBI | CHEBI:132999 |
| ChEMBL | CHEMBL427907 |
| ChemSpider | 10618 |
| DrugBank | DB14004 |
| ECHA InfoCard | echa.europa.eu/infocard/100.007.864 |
| EC Number | EC 206-190-0 |
| Gmelin Reference | 94536 |
| KEGG | C02597 |
| MeSH | D013845 |
| PubChem CID | 9647 |
| RTECS number | YN8225000 |
| UNII | HM4YQM8Y6D |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | 4887 |
| Properties | |
| Chemical formula | C4H8O4S2 |
| Molar mass | 154.18 g/mol |
| Appearance | Colorless crystals or liquid |
| Odor | Odorless |
| Density | 1.46 g/cm³ |
| Solubility in water | Soluble |
| log P | -1.21 |
| Vapor pressure | <0.01 mmHg (20 °C) |
| Acidity (pKa) | 2.87 |
| Basicity (pKb) | 2.10 |
| Magnetic susceptibility (χ) | -63.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.583 |
| Viscosity | Viscosity: 3.98 mPa·s (at 20 °C) |
| Dipole moment | 2.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 229.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -818.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1067.8 kJ/mol |
| Pharmacology | |
| ATC code | V03AB37 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and serious eye irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05 |
| Signal word | Danger |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: P280, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 2-1-2 |
| Flash point | 185 °C |
| Autoignition temperature | 180°C |
| Lethal dose or concentration | LD50 oral rat 2850 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 2.48 g/kg |
| NIOSH | NT8050000 |
| PEL (Permissible) | PEL 15 mg/m3 |
| REL (Recommended) | 0.02 mg/L |
| IDLH (Immediate danger) | Not Established |
| Related compounds | |
| Related compounds |
Thioglycolic acid
Oxalic acid Glycolic acid Thiodiglycol Sulfuric acid |
