Thiourea: A Detailed Look at an Unassuming Chemical
Historical Development
Thiourea’s roots stretch back to the 1800s, with the tale of its discovery reflecting the curiosity and experimental drive of early chemists. Around 1821, German and French researchers started working with urea derivatives and noted the differences brought by substituting oxygen with sulfur, giving birth to thiourea. In the decades that followed, industrial processes evolved in sync with textile and agricultural growth. Companies across Europe and later in Asia turned to thiourea for its role as a reducing agent, and over time, large-scale usage in mining and photographic industries became common. Looking at production numbers, the late 20th century marked a phase where thiourea moved out of academic labs and into mass-production, with thousands of tons shipped annually.
Product Overview
On its own, thiourea comes as a white crystalline powder with a distinctly bitter taste. Chemists and engineers value it because it dissolves easily in water, forming transparent solutions. It doesn’t deliver much aroma in its raw form, but it surprises many by how it reacts with a wide range of metal ions. Industrial catalogs list it under various grades, but all exhibit similar structure — simple, robust, and able to act as a strong nucleophile.
Physical & Chemical Properties
Thiourea’s molecular formula, CH4N2S, hints at its simplicity. Its melting point sits near 180°C, but under heat, it doesn’t burn in the classic sense. Instead, it decomposes and releases toxic gases, mostly sulfur compounds that irritate eyes and lungs. Water grabs onto thiourea quite well, yielding almost 140 grams per liter at room temperature. Some solvents handle it poorly — organic non-polars keep it out. With a density around 1.4 grams per cubic centimeter, it packs surprisingly well. Chemically, thiourea stands out for its ability to coordinate with metals, acting as a ligand in many complexes.
Technical Specifications & Labeling
Buyers often demand tight specifications: purity over 99%, limits on heavy metal content, and strict particle size distributions. Labels highlight storage requirements: cool, dry locations, containers kept tightly sealed. GHS pictograms warn of acute toxicity. Most suppliers provide clear batch numbers and testing certificates. Technical data sheets focus on solubility figures, stability in air, and restrictions for food or pharmaceutical uses. Agencies like OSHA and REACH have placed reporting demands, and reputable vendors must carry up-to-date safety datasheets.
Preparation Method
Industrial synthesis usually relies on the reaction of cyanamide and hydrogen sulfide under controlled temperatures. Older methods sometimes used ammonium salts reacting with calcium sulfide, but handling the resulting sulfide waste and gas release turned out to be impractical at scale. Modern reactors, made of corrosion-resistant alloys, allow operators to push yields above 80% in semi-batch processes. Firms investing in sustainable chemistry now seek recycled feedstocks, slashing the environmental toll compared to mid-century practices.
Chemical Reactions & Modifications
The sulfur atom in thiourea attracts researchers who work with both organic and inorganic synthesis. Thiourea forms strong complexes by donating electrons through nitrogen and sulfur. In gold leaching, it provides an alternative to cyanide, grabbing gold ions and allowing extraction without the notorious hazards of cyanidation. Organic chemists use it to introduce sulfur into rings or to reduce a wide range of functional groups, converting ketones back to alcohols and changing halides into thiols. Modifications through alkylation or acylation produce derivatives used as pharmaceuticals or agrochemicals.
Synonyms & Product Names
Thiourea does not just hide behind one name. In chemical registries, one finds synonyms such as thiocarbamide, sulfourea, and even “pseudothiourea” from older European labels. Its CAS number, 62-56-6, provides a unique way to source high-purity stocks across the globe. Some commercial brands use subtle changes in spelling, but the backbone composition remains identical. Researchers become familiar with these synonyms from scanning literature spanning the last two centuries.
Safety & Operational Standards
Handling thiourea safely takes more diligence than many realize. It doesn’t ignite with a spark, but that property leads to false confidence. Dust can irritate the respiratory tract and eyes, and skin absorption leads to allergic reactions in rare cases. Chronic exposure triggers thyroid changes. Occupational standards, like those from OSHA and EU-REACH, limit permissible air concentrations to tiny fractions of a milligram per cubic meter. Plant operators enforce ventilated work spaces, issue gloves and splash goggles, and train teams to handle accidental spills with sturdy absorbents. Proper signage and chemical storage guidelines stand as non-negotiable rules.
Application Area
Thiourea finds buyers in oddly diverse fields. Gold mines in Asia and Africa use it to recover precious metal from ore without the scandal and environmental damage of cyanide. In agriculture, it accelerates seed germination and breaks potato dormancy, giving farmers tighter harvest cycles. Textile processing lines depend on its reducing properties for dyeing. Photographers in the pre-digital area used thiourea in silver recovery and toning solutions. Research labs pick thiourea for synthesizing heterocyclic compounds, while specialty chemical manufacturers build more complex drugs and crop-protecting chemicals from simple thiourea blocks.
Research & Development
Academic labs worldwide treat thiourea as a base chemical for experimenting with sulfur transfer reactions and metal complexation. Over the past decade, green chemistry researchers have developed more energy-efficient methods to recycle and reuse thiourea leachates from mining. New applications keep emerging; papers published this year describe photovoltaic cells relying on thin films generated from thiourea precursors. Pharmaceutical firms have invested in modifying the core thiourea to produce antiviral compounds and thyroid drugs. The research landscape is lively, reflecting both the chemical’s versatility and the evolving needs of industry and medicine.
Toxicity Research
Most discussions about thiourea turn cautious when the subject shifts to toxicity. In animal models, thiourea disturbs thyroid function, acting as a goitrogen by disrupting the uptake of iodine. Epidemiological studies of exposed workers highlight slight but significant changes in hormone levels after long-term exposure. Regulatory science keeps pushing for more rigorous monitoring and occupational medical checks. Safety data guides handling practices—from limiting dust exposure to engineering closed transfer systems. The EU has listed it under substances of very high concern, forcing firms to disclose usage and support worker health studies.
Future Prospects
Looking forward, the fate of thiourea will depend on mining reform, agricultural modernization, and the relentless search for cleaner and safer chemicals. R&D in green metals extraction and sustainable food production might cement thiourea as an alternative where legacy options fail the safety or environmental test. As photovoltaic and advanced material sectors expand, new uses for organosulfur compounds could add demand. Meanwhile, pending regulations in the EU and North America highlight the need for greener production and stricter controls for waste and worker exposure. The next chapter for thiourea stands to be written by innovators willing to tackle health and sustainability challenges without giving up performance.
Everyday Uses of Thiourea
Walk into a photography darkroom or leaf through old textbooks on ore processing, and thiourea makes regular appearances. This sulfur-containing compound earned its reputation because it gets involved in processes where few alternatives step up. In photography, it removes silver halides from negatives, letting images develop clearly. Many of the best snapshots from decades ago owe their clarity to a solution laced with thiourea.
Jewelry makers value thiourea for more reasons. It plays a key role in stripping gold and silver from old surfaces and in refining precious metals. As someone who once watched a craftsman bring dull jewelry back to life, I've seen how thiourea helped clean prongs and settings without eroding too much metal. Waste gets minimized, and the piece glows like new.
Applying Thiourea in Industry
Factories and chemical plants need thiourea to create other chemicals. Textile workers depend on it during dyeing, especially when working with synthetic fibers. A friend in the textile industry pointed out that getting vibrant colors and keeping fabric from fading would be much harder without this compound. Dyers and finishers rely on chemicals that don’t break the bank yet deliver strong results, and thiourea fits that bill.
Pulp and paper mills add small amounts of thiourea when bleaching paper. It helps prevent yellowing and keeps paper sheets clean and white. Companies add it to anti-corrosive coatings and metal cleaners, often as a safer option compared to stronger, harsher acids.
Health, Safety, and the Environment
Safety matters to families, and not many folks realize thiourea has some rough edges. Chronic exposure can cause health problems; regulatory agencies suggest clear safety guidelines. It’s classified as a possible carcinogen by the International Agency for Research on Cancer (IARC). Keeping contact low and using proper gear when handling it should be a strict rule, especially for workers who breathe in dust or handle liquids for hours.
Environmental concerns also enter the picture. Thiourea does not break down quickly in water or soil, so runoff can linger and affect fish and plants. Local regulators and community activists should pay attention to discharge records. Regular checks along rivers or near landfill sites can signal early warning signs before problems get out of hand.
Finding Solutions and Moving Forward
Cleaner manufacturing offers a way forward. Companies can invest in better treatment systems, swapping out older equipment and catching spills before they spread. Engineers can adjust processes to minimize thiourea use. In the lab, teams look for safer options—biodegradable catalysts or plant-based substitutes show promise, though results take time.
Teachers can do their part too. By including lessons on chemical safety and waste in classrooms, students develop respect for powerful compounds. A step like this, repeated over decades, might reduce accidents and spark fresh ideas for future chemists and inventors.
Thiourea certainly packs a punch in more places than people realize. Treating it with a mix of respect, caution, and curiosity can protect workers, neighbors, and the streams running behind our schools and homes.
Everyday Interactions With Chemical Risks
Thiourea rides under the radar for most people, yet it plays a role in many manufacturing sectors—photography, mining, textile dyeing, and even in lab research. I spent years teaching basic industrial safety, often shocked by how little folks understand about common shop chemicals, thiourea included. A name without reputation still deserves a hard look.
The Science of Danger
Thiourea is not just any industrial additive. Research pegs it as a possible carcinogen. Studies raise red flags about its link to thyroid issues and cancer development in rodents, and that alone stops me from handling it carelessly. The International Agency for Research on Cancer classifies it as possibly carcinogenic to humans (Group 2B). That means, from a safety perspective, you treat it as suspicious, not innocent until proven guilty.
Acute exposure paints another bleak picture. Inhaling thiourea dust or vapors—or skin contact—often leads to irritation. It can hit the respiratory tract, redden the eyes, inflame the skin, and trigger allergic reactions. Longer-term or repeated exposure has been tied to issues with the thyroid gland, worsening the worry for people working with the chemical day after day.
Lessons From Worksites and Research Labs
I learned early on that you can't tell how hazardous a chemical is just by looking at it. Adequate training and strict personal protective equipment policies stand as the only defense. I've seen facilities let safety slip and wind up facing costly fines—or worse, real health tragedies. The difference lies in respect for the risk. Proper gloves, protective eyewear, lab coats, and solid ventilation systems matter. The workers know it, the managers should too, yet shortcuts linger in the name of saving time.
Environmental Concerns
Beyond immediate health risks, thiourea brings up another topic—impact on water systems. It breaks down slowly in nature. If it enters rivers or soil, it could affect aquatic life and drinking water, creating headaches for communities downstream of factories. Combating this issue requires strict industrial waste management and regular environmental monitoring. I've witnessed communities demand action after discovering nearby chemical leaks, and their voices shape regulations today.
Better Awareness, Smarter Choices
One fix sits in greater awareness. Workers benefit from safety briefings that don't just tick boxes but genuinely explain risks and symptoms. Companies need real monitoring: air quality checks and medical screening for those regularly exposed. Tighter labeling and warning systems on chemical containers also help.
I encourage workers, managers, and regulators to keep up momentum on chemical safety—in every industry, not just the headline-makers like pesticides or asbestos. Trust grows from hard data and good training, not wishful thinking. Those who underestimate thiourea often do so out of ignorance, not malice.
Finding a Safer Path Forward
Substitution offers another option. If safer chemicals do the job, use them. Researchers constantly hunt for alternatives with less risk. Until then, respect the potential harm and ensure protective measures are in place. That’s the safest bet for companies, communities, and the environment alike.
Discovering Thiourea’s Make-Up
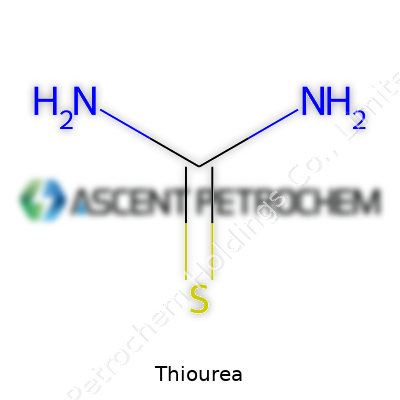
Thiourea stands out in my memory from chemistry class as a compound with a straightforward yet meaningful structure. Its chemical formula is CH4N2S. This formula isn’t just a bunch of letters and numbers. It shows hydrogen, carbon, nitrogen, and sulfur working together in a molecule that continues to matter in real-world practices.
Breaking Down CH4N2S
Most people rarely think about where the things they use every day come from or what goes into making them. Thiourea brings together one carbon atom, four hydrogens, two nitrogens, and one sulfur atom. Compared to urea, it swaps out an oxygen atom for sulfur, giving it some useful properties. This touch of sulfur brings relevance in certain industries, including agriculture, textiles, and even photography. It isn’t the most eye-catching molecule on the shelf, but anyone who has worked in a lab or a plant knows chemistry’s workhorse value.
Real-World Use Makes a Difference
Seeing thiourea on a chemical container, I would remember research mishaps and the sharp odor that seemed to follow it around the lab. Farmers and industrial engineers rely on this compound every season. In fertilizer formulations, adding thiourea gives a source of nitrogen and sulfur, nutrients that crops need to thrive. Crops lacking these elements don’t just slow down; they stunt and fade, leading to smaller harvests. This in turn threatens food security and the livelihoods of millions of people who rely on agriculture.
Textile workers use thiourea for dyeing and printing fabrics. Without it, certain dyes don’t set well, colors fade, and the process gets less efficient. It plays a quiet but serious part in making sure shirts hold onto their pattern after dozens of wash cycles.
Photographers in the analog era depended on thiourea to tone prints, shifting the shadows in a piece to give it character and life. Even now, with digital images everywhere, artisan printmakers go back to the chemistry that comes from molecules like CH4N2S to craft something unique.
Navigating Challenges and Looking Forward
Some plants have run into trouble from thiourea misuse, especially cases involving over-application in the fields. This can harm both plants and soil, making responsible use key. Regulatory groups and advisory bodies focus on best practices to guard against misuse and accidental exposure. Farmers and workers need up-to-date training, easy-to-understand safety sheets, and better access to personal protective gear to keep themselves and the environment healthy.
From my own experience, transparency and knowledge-sharing between chemists, industry leaders, and the people using these chemicals matter more than any single regulation. Research teams must continue working on greener ways to do the same jobs, so useful chemicals like thiourea don’t risk turning into unwanted environmental burdens over time.
Investing in Safe, Responsible Chemistry
Even a tiny molecule like thiourea can play a big role. Knowing its formula, knowing where it ends up, knowing how it interacts with the fields, the rivers, and the food we share—these are facts that help us all decide how to work with care and confidence. Responsible chemistry builds trust from the ground up, one simple formula at a time.
Why Storage Choices Matter for Thiourea
I’ve spent years around chemical storage rooms. Every time Thiourea comes up, there’s a bit of tension in the air. People know it helps out in many processes—photography, gold extraction, industrial synthesis—but the real challenge always shows up when it’s time to keep it safely tucked away.
Humidity: The Silent Spoiler
Moisture causes most of the headaches. Thiourea can clump and lose quality with just a little water in the air. Pretty much everyone I talk to agrees on using tight-sealing containers. Plastic buckets with locking lids and heavy glass jars with rubber gaskets work better than cheap snap-top containers. A dry storage spot, locked away from water lines or humid basements, protects the batch.
Look: Even a good storage setup can stumble when the location stays damp. Keeping packets of desiccant in the jar adds a safety net. I’ve seen labs get lazy and toss desiccant on the shelf instead of inside the container, and by the time somebody realizes, the thiourea picks up moisture anyway.
Heat: The Enemy of Longevity
Heat speeds up chemical changes you really want to avoid. No one stores solutions like thiourea next to heat sources, yet mistakes happen—storage next to boilers, windows, or power equipment shortens shelf life and sometimes even nudges dangerous reactions along. A cool, shaded storage room pays off every time. The American Chemical Society points toward 15°C to 25°C as a sweet spot.
Chemical labels spell it out, but people get distracted. I like to set up wall thermometers in storage areas as a simple reminder. Walking in and seeing the temperature too high means it’s time to move the chemicals, no excuses.
Light: What People Overlook
Thiourea handles most room-light, but fierce sunlight will do a number on it over time. I’ve cracked open more than one container that sat on a shelf in sunny spots only to find yellowed, degraded powder. Sure, the window makes the room pleasant, but chemical storage needs dull, shaded areas.
Wrapping containers in brown paper or putting them in colored bins gives cheap extra protection if shelf space gets tight. It’s not elaborate, but field fixes like these keep supplies safe until more permanent storage appears.
Labeling and Inventory Are Key
It’s easy to forget what’s inside a nondescript container, especially once the labels start to peel. I see mistakes with thiourea mix-ups all the time—sooner or later, somebody uses the wrong material for an experiment. Clear labels protect everyone and reduce expensive errors.
Inventory sheets play a bigger role than most folks realize. No one wants to discover a forgotten batch that’s decayed past safe use. Ticking off entries regularly keeps the whole workplace safer.
Access and Security Count
This stuff deserves a locked cabinet. Too many times, unauthorized folks wander through labs or workshops and help themselves. Under lock and key, with access logs, accidents and theft fall away. Most organizations link chemical control to work permits or staff lists, but sometimes, busy days lead to shortcuts. Serious oversight stops those steps from being skipped.
Personal Experience and Responsibility
Everyone storing thiourea owes it to their coworkers to double-check storage conditions. Regular walkthroughs catch little mistakes before they turn into health problems or wasted stock. It’s not just about following a checklist; it’s about creating habits that make safety automatic.
People learn quickly if they see their own actions improving a workspace. Stories of lost batches or close calls with thiourea reach new team members fast. Sharing those lessons makes sure fewer slip-ups happen as the years go by.
The Backbone Chemical in Mining
In mining, especially during gold and silver extraction, thiourea steps in when traditional methods struggle or hit environmental snags. It acts fast, latching onto precious metals and letting miners pull more value from stubborn ore. Gold leaching with thiourea may cost more than the classic cyanide process, but some mines will pay for it if it helps them reach tricky deposits or keeps them within modern safety rules. I’ve spoken with geologists who value its reliability in stubborn terrain, where every gram counts and government rules keep shifting.
Photography and Its Chemical Roots
Before the digital age, darkrooms held their own kind of magic—and a whole lot of chemistry. Thiourea plays a key part in toning photographs, helping black-and-white prints last longer while tweaking their tones from gray to sepia. Even now, some artists and specialist labs stick with thiourea toning for the unique look and extended shelf-life it gives their work. That’s a hands-on field with tight safety habits, since handling chemicals day in and out makes folks more careful about exposure and disposal.
Fabric Treatments and Textiles
Clothing manufacturers count on thiourea, too. Cotton goes through plenty of steps before it ends up as a shirt or sheet—bleaching, dyeing, finishing. Thiourea helps stabilize certain dyes and keeps colors locked in, bright after many washes. It’s not uncommon for dye houses to use its reducing power to set prints or prep fibers. Some fabric engineers told me they appreciate how it can save on water and energy, which matters as the world keeps asking for cleaner, more efficient manufacturing. Meanwhile, workers in these plants need steady training and gear, since long-term chemical contact can be rough on skin and lungs.
Paper Takes a Boost
Papermaking plants mix in thiourea during pulp bleaching. The chemical softens lignin and turns out a brighter sheet that feels better in your hands and prints cleaner images. The paper world moves fast these days, but thiourea lets them reach for higher brightness without heavy chlorine use. That cuts down on harsh waste—something everyone within earshot of a mill can support, since river and air quality stay in the news. I’ve toured a few mills that pride themselves on finding ways to blend tradition and new chemistry to keep the EPA satisfied and workers healthy.
Pesticides and Pharmaceuticals
On the farm, thiourea-based products help fight fungus and keep crops in better shape. In the lab, chemists use it to build all sorts of compounds, especially in drug discovery. That means thiourea crops up in medicines and agrochemicals—places where tight oversight and deep testing matter. Conversations I’ve had with chemical engineers back up the push for lower exposure and smart containment, since impurities can seep into the food chain if no one pays attention.
Ways Forward and Takeaways
People often overlook how deeply chemistry affects manufacturing and daily life. Thiourea carries risk and benefit, depending on how companies handle sourcing, workers, and the planet. Best practices focus on real-time monitoring and process tweaks, while new research looks at safer alternatives or recycling spent thiourea streams. Factories and labs need up-to-date safety training. The public can push for more transparency, so everyone knows the impact of the chemicals tied to their gold rings, photographs, cotton shirts, or medicines.
| Names | |
| Preferred IUPAC name | thiourea |
| Other names |
Thiocarbamide
Thiourea dioxide Isothiourea |
| Pronunciation | /ˌθaɪ.əˈjʊə.ri.ə/ |
| Identifiers | |
| CAS Number | 62-56-6 |
| 3D model (JSmol) | `load =3d6s` |
| Beilstein Reference | 3490818 |
| ChEBI | CHEBI:28936 |
| ChEMBL | CHEMBL1409 |
| ChemSpider | 5469 |
| DrugBank | DB04653 |
| ECHA InfoCard | 03b14433951a-48a7-93f7-3b2e77deb1fa |
| EC Number | 'EC 202-197-6' |
| Gmelin Reference | 62129 |
| KEGG | C01352 |
| MeSH | D013958 |
| PubChem CID | 1130 |
| RTECS number | XK6650000 |
| UNII | GNQ1C3IM1Z |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID5023842 |
| Properties | |
| Chemical formula | CS(NH2)2 |
| Molar mass | 76.12 g/mol |
| Appearance | White crystalline solid |
| Odor | Odorless |
| Density | 1.41 g/cm³ |
| Solubility in water | 137 g/100 mL (20 °C) |
| log P | -1.61 |
| Vapor pressure | 0.04 mmHg (20°C) |
| Acidity (pKa) | 21.1 |
| Basicity (pKb) | 11.0 |
| Magnetic susceptibility (χ) | -44.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.590 |
| Viscosity | 1.41 mPa·s (20 °C) |
| Dipole moment | 4.02 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 115.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -107.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -475.6 kJ/mol |
| Pharmacology | |
| ATC code | V03AB02 |
| Hazards | |
| Main hazards | Harmful if swallowed or inhaled. Suspected of causing cancer. Causes skin and eye irritation. |
| GHS labelling | GHS02, GHS06, GHS08 |
| Pictograms | GHS06,GHS08,GHS09 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Suspected of causing cancer. Causes damage to organs through prolonged or repeated exposure. Very toxic to aquatic life. |
| Precautionary statements | P210, P261, P273, P280, P302+P352, P304+P340, P312, P321, P330, P363, P501 |
| NFPA 704 (fire diamond) | 1-2-0-Acidity |
| Autoignition temperature | 410 °C (770 °F; 683 K) |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 (oral, rat): 1,250 mg/kg |
| LD50 (median dose) | LD50 (median dose): 175 mg/kg (oral, rat) |
| NIOSH | WN2975000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Thiourea: "5 mg/m3 (OSHA, 8-hour TWA) |
| REL (Recommended) | 13 mg/m³ |
| IDLH (Immediate danger) | 100 mg/m3 |
| Related compounds | |
| Related compounds |
Urea
Thioacetamide Guanidine Isothiourea Mercaptobenzothiazole Dithiocarbamate |
